�ס��ҡ��������ֵ��ʣ������������壬���dz����ĺ�ɫ���壬A��B��C�����ֻ��������֮��������ת����ϵ��

��1���û�ѧʽ��ʾ�������ʣ�B
CuO
CuO
��
H2
H2
��
C
C
��2��д���ݷ�Ӧ�Ļ�ѧ����ʽ��
��
��3�������ݵķ�Ӧ�����ڸ��ֽⷴӦ����
��
��
������ţ���
��4��ij����С��������������B��Ӧ�ⶨCu�����ԭ����������ͼ�Dzⶨװ�õ�ʾ��ͼ��E�е��Լ������ᣮ����֪�Ȼ���������ˮ����ʯ����һ�ָ��������ش��������⣮

��a��������װ����Լ� F
CuO
CuO
��G
H2O
H2O
��H
Ũ����
Ũ����
��
��b�����Ӻ�װ�ú�Ӧ����
���װ�õ�������
���װ�õ�������
���䷽����
�ر�E�еĻ�������K��ܽ�û��ʢ��ˮ���ձ��У�������ƿF���۲�K�ܿڣ����������ݳ���˵��װ�õ�����������
�ر�E�еĻ�������K��ܽ�û��ʢ��ˮ���ձ��У�������ƿF���۲�K�ܿڣ����������ݳ���˵��װ�õ�����������
��c����Ӧ������K���ݳ���������
H2
H2
��
��d����ʵ���в������������
�ٿ�I�ܵ�����a
�ڷ�Ӧ��I�ܺ�Cu�۵�������c����ȴ�����³�����
�۷�ӦǰJ�ܼ���ʢ���������d
�ܷ�Ӧ��J�ܼ���ʢ���������e
��������������г�Cu�����ԭ�������ı���ʽ����Cu�⣬�����漰��Ԫ�ص����ԭ��������Ϊ��֪����
A
r��Cu��=
��






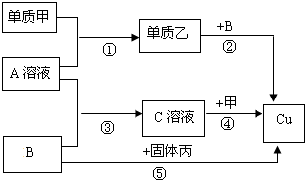 �ס��ҡ��������ֵ��ʣ������������壬A��B��C�����ֻ��������֮��������ת����ϵ��
�ס��ҡ��������ֵ��ʣ������������壬A��B��C�����ֻ��������֮��������ת����ϵ��
 26����֪A��B��C��D�����ֻ�����ס��ҡ��������ֵ��ʣ�A�Ǻ�̼���A����Է�������С��20����һ�������£�����֮���������ת����ϵ��
26����֪A��B��C��D�����ֻ�����ס��ҡ��������ֵ��ʣ�A�Ǻ�̼���A����Է�������С��20����һ�������£�����֮���������ת����ϵ��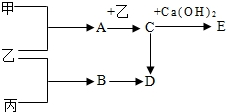 �ס��ҡ��������ֵ��ʣ����м��ǹ��壻�ҡ��������壻A��B��C��D��E���ǻ�����ܶཨ��������E���ܵ��������ʴ���������ʵ��ת����ϵ����ͼ��ʾ�����ֲ�������ȥ����
�ס��ҡ��������ֵ��ʣ����м��ǹ��壻�ҡ��������壻A��B��C��D��E���ǻ�����ܶཨ��������E���ܵ��������ʴ���������ʵ��ת����ϵ����ͼ��ʾ�����ֲ�������ȥ����