
| ������Һ���¶�/�� | ��75 | ��65 | 50 | 35 | ����20 |
| ��������M������/g | ����0g | ��0g | 2.0g | 4.5g | 8.4g |
���� ����Ŀ����Ϣ��֪��75��ʱ������Һ�ǣ����DZ�����Һ����40��ʱ�ӽ����͵�M��Һ��ɱ�����Һ��һ���ܴﵽĿ���У�������M����������ˮ��20��ʱ���ù����ĩM��ˮ����100g������������Ϊ5%��M��Һ�������õ��������У�������ƽ��������У����ձ���ҩ�ס���Ͳ��100mL������ͷ�ιܣ���������
��� �⣺��1������Ŀ����Ϣ��֪��75��ʱ������Һ�ǣ����DZ�����Һ����Ϊ���º�û�й����������ʴ�Ϊ�����DZ�����Һ��
��2����40��ʱ�ӽ����͵�M��Һ��ɱ�����Һ��һ���ܴﵽĿ���У�������M����������ˮ���ʴ�Ϊ���ۢݣ�
��3��20��ʱ���ù����ĩM��ˮ����100g������������Ϊ5%��M��Һ�������õ��������У�������ƽ��������У����ձ���ҩ�ס���Ͳ��100mL������ͷ�ιܣ����������ʴ�Ϊ����������
���� ���⿼�����жϱ�����Һ�벻������Һת���ķ����������ܽ�����¶����߶���������ʣ��ɲ�������Һ��ɱ�����Һ�ķ����ȣ���������Ҫ������ѡ�����������У�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
| ������������ | 2% | 2% | 2% | 6% | 6% | 6%] | 10% | 10% | 10% |
| ˮ���¶ȣ��棩 | 20 | 40 | 60 | 20 | 50 | 60 | 20 | 40 | 60 |
| ��ҺpH | 10.90 | 11.18 | 11.26 | 11.08 | 11.27 | 11.30 | 11.22 | 11.46 | 11.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 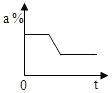 | B�� | 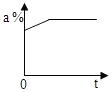 | C�� | 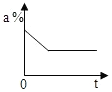 | D�� | 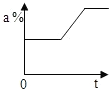 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 |  |  |  |
| A����һƿŨ����¶���ڿ����� | B����һ��������ϡ�����м���пƬ | C������һ�������ĸ�����ع��� | D����һ���������Ȼ�þ�� ϡ����Ļ����Һ����μ�������������Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
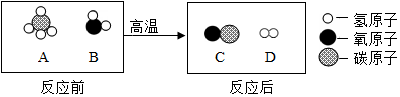
| �¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� ��g/100g ˮ�� | KNO3 | 13.3 | 31.6 | 63.9 | 110.0 | 169.0 | 246.0 |
| NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | ���� | ��ȥ���ʵķ��� | |
| A | CaO | CaCO3 | ����������ˮ�ܽ⡢���� |
| B | NaOH��Һ | Na2CO3 | ����������Ca��OH��2��Һ������ |
| C | Cu��NO3��2��Һ | AgNO3 | ���������ͭ�ۣ����� |
| D | CO | CO2 | ͨ��NaOH��Һ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
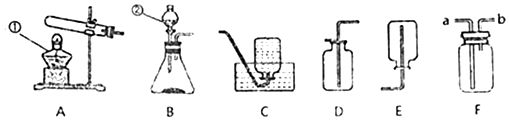
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com