
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
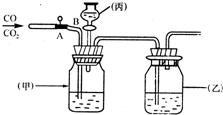
 10��С����ȡ���Ļƺ�ˮ��Ʒ�����ձ��У����ú�����ͼ��ʾװ�ý��й��ˣ����ж�����˵���д�����ǣ�������
10��С����ȡ���Ļƺ�ˮ��Ʒ�����ձ��У����ú�����ͼ��ʾװ�ý��й��ˣ����ж�����˵���д�����ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
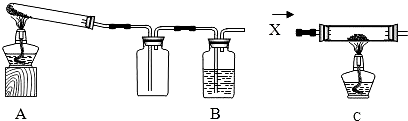
| װ�� | ��Ӧǰ | ��Ӧ�� |
| A | �Թܵ�����38.2 �� ����ͭ��̿�ۻ���������20.0�� |
�Թܺ������ʵ�����56.8 �� |
| B | ��Ӧ��ƿ��ʯ��ˮ�ȷ�Ӧǰ����1.1 �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��Ⱦָ�� | ��Ҫ��Ⱦ�� | ������������ | ��������״�� |
| 55 | S02 | �� | �� |
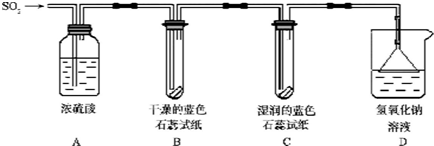

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
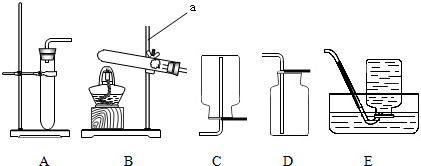
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com