
����̼�����һ����Ҫ����������Ʒ����ͼ��ij����������̼��ƵĹ������̡�
��ش��������⣺
��1������ɲ���ƽ�ù���������CaCO3����ķ�Ӧ����ʽ��
CaCl2+_________+CO2 CaCO3��+__________+H2O
CaCO3��+__________+H2O
��2������ʵ�����н��и�ʵ�飬����l�������� ��ʹ�õ��IJ��������в�������________��_________�����в������������� ��
��3������2Ϊϴ�ӡ��������ϴ�ӵ�Ŀ���dz�ȥ̼��ƴ�Ʒ���溬�еĿ��������ʣ�����һ�����еĿ����������� �������Ƿ�ϴ�Ӹɾ��ķ����������һ��ϴ��Һ�м��� ��Һ����Ca(OH)2 ���� AgNO3���� CaCl2����д��ţ������û�г��ֳ�����˵���Ѿ�ϴ�Ӹɾ���
��4�����������еĸ���Ʒ��NH4Cl��������_____________________��(дһ����;)
��1��2NH3��H2O 2NH4Cl ��2������ �ձ���©�� ����
��3��NH4Cl �� ��4�����ʣ����ʡ����ϣ�
���������������1��������������̼��ƵĹ�������ͼ��֪���������NH3��H2O��Ҳ����������һ�ַ�Ӧ�ͬʱ��������NH4Cl���ٸ��������غ㶨�ɵ��۽��ͣ����Է�Ӧ����ʽ��CaCl2 +2NH3��H2O + CO2  CaCO3�� +2NH4Cl + H2O
CaCO3�� +2NH4Cl + H2O
��2������l�ǽ�������Һ����룬�����ǹ��ˣ���ʹ�õ��IJ����������˲���������Ҫ�ձ���©�������в������������ǣ�����
��3������2Ϊϴ�ӡ��������ϴ�ӵ�Ŀ���dz�ȥ̼��ƴ�Ʒ���溬�еĿ��������ʣ�����һ�����еĿ��������ʣ���ʵ���Ƿ�Ӧ��������Һ�е����ʣ���NH4Cl������Ϊ�˼����Ƿ�ϴ�Ӹɾ���ֻҪ֤�����һ��ϴ��Һ�Ƿ���NH4Cl���ʷ����������һ��ϴ��Һ�м�AgNO3�����Ƿ��а�ɫ�������֣����û�г��ֳ�����˵���Ѿ�ϴ�Ӹɾ�����ѡ��
��4��NH4Cl�к�Ӫ��Ԫ��N�����Կ�������
���㣺�����غ㶨�ɵ�Ӧ�ã����˲���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ����ԭ�ӵĽṹʾ��ͼ��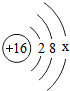
��1��ͼ��x=�� ������������ ������������ǽ�������Ԫ�أ�
��2������������ȼ�շ����� �������Ӧ�Ļ�ѧ����ʽ���� ����
��3����ȼ�ճ���ȼ�ŵ������ˮ�У�ʹ��Ϩ���ԭ������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�û�ѧ������գ�
��Ԫ��____________��2��������___________��������________��ˮ����Ԫ�صĻ��ϼ�____________��ľ̿ȼ�յĻ�ѧ����ʽ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(3��)��ѧ������������������ء�
��1������ũҩ������Һ��������ͭ�Ĺ��������� ���ѧ���ţ�
��2���ƾ�ȼ�յĻ�ѧ����ʽΪ ��
��3���ȼҵ�е��ʳ��ˮ�õ��ռ���������壬һ������������壬��һ�ֵ���Է�������Ϊ71���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��Ӳˮ�к��϶��Ca2����Mg2�������� ����Ӳˮ����ˮ����84������Һ����Ч�ɷ�NaClO����Ԫ�صĻ��ϼ�Ϊ ��Cl2��������ˮ����������ʵ�����Ʒ�Ϊ��MnO2��4HCl��Ũ�� �� X��Cl2����2H2O��X�Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
���û�ѧ����ʽ��ʾ�����йط�Ӧ��ԭ����
��1������������Ȼ����ȼ�ϣ�
��2����������⣮
��3������̼��������θ����࣮
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��ѧ��һ��˫�н�����ѧ�������������ܸ��õ�Ϊ���Ƿ���
��1�����û�ѧת���ķ���������������ʵ������ʺ;��ü�ֵ���磺�����۵���ʯ��������еĴ��Ӧ���Ϳ�����ȡ��;�㣮�۸�ߵ��ռ�仯ѧԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��2����ѧ��Ʒʹ�ò�����Ҳ����������Σ�գ��磺2013��5��4�գ����ӡ������̸�����ع���ʡij�ز������þ綾ũҩ��������ũ������ֲ�����ʹ�е�Υ����Ϊ����ũҩ�Ļ�ѧʽΪC7H14N2O2S������ ��Ԫ����ɣ�����Է�������Ϊ ����������������Ԫ�ص�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��8�֣�Ϊ̽�������֮��ķ�Ӧ��С��ͬѧ��������ʵ�飺��ϡ�����м�����һ���������ᱵ��Һ����ش�
��1���۲쵽��ʵ�������� ����Ӧ�Ļ�ѧ����ʽΪ ������ʵ��������Һ�м��ٵ������� ��д���ӷ��ţ���
��2��С��ͬѧ��ϡ�����м������ᱵ��Һʱ�����ᱵ��Һ���ܹ�����������Һ�к�������
���ᱵ�ķ����� ��
��3����98g��������Ϊ10%��ϡ�����м���100g���ᱵ��Һ��ǡ����ȫ��Ӧ���������ᱵ��Һ�����ʵ����������� ����������ȷ��0��1%����
��4����Ҫ����98g��������Ϊ10%��ϡ���ᣬ��Ҫ��������Ϊ98%��Ũ���ᣨ�ܶ�Ϊ
1��84g/cm3�� mL����������ȷ��0��1������ʵ������Ũ��������ϡ�������Ҫ�����У����㡢 �����ȡ���ȴ������װƿ�������ϱ�ǩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��7�֣�����һ��������A�ijɷ֣���֪���п��ܺ���NH4C1��Na2SO4��CuCl2��NaCl��AgCl���������е����ֻ���֡�����ͼ��ʾ����ʵ�飬���ֵ���������ͼ������������������п��ܷ����ķ�Ӧ��ǡ����ȫ��Ӧ����
�Ը���ʵ����̺ͷ�����������д���¿հף�����д��Ӧ�Ļ�ѧ���ţ���
��1�������A�У�һ�������ڵ������� �����ܴ��ڵ�������
��2������B�� ,����XΪ
��3����ҺC�У����ڵĽ�����������
��4������D�� ��
��5��д��ʵ����̢��з����Ļ�ѧ��Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ο��������ʵ��۽ṹͼʾ�����������ӹ��ɵ�������
| A��ͭ | B���ɱ� | C���Ȼ��� | D�����ʯ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com