�ڡ���������ȡ�����ʡ�ʵ����У�ijͬѧȡһ��ɴ�����ϵ�ϸ��˿�����Լ��ռ�����������������˿��������ȼ�ա���ʵ�飮���û�й۲쵽���������䡱�������������ʵ��ʧ�ܵ�һ�ֿ���ԭ��
��˿�Ѿ����⣨���ռ��������������¶�û�дﵽ�����Ż���
��˿�Ѿ����⣨���ռ��������������¶�û�дﵽ�����Ż���
�������Ľ�ʵ��ɹ��ˣ��䷴Ӧ�Ļ�ѧ����ʽΪ
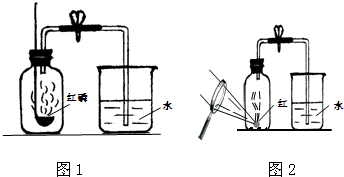
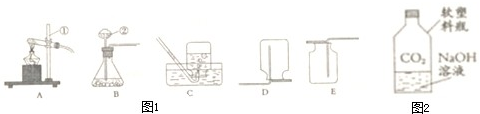
�������ⶨ�����ɷֵķ����ܶ࣬��ͼ1��ʾ�����ú����ڿ�����ȼ�յIJⶨ������ʵ������ǣ�
��һ����������ƿ�ݻ�����Ϊ��ȷݣ������ñ�ǣ�
�ڶ�������ȼȼ�ճ��ڵĺ��ף����뼯��ƿ�в�������������
��������������Ϩ����ȴ���ɼУ�����ˮ�����뼯��ƿ�У����뼯��ƿ��ˮ�����ԼΪ����ƿ���ݻ���
��
��ش��������⣺
��1������ƿ��ʣ���������Ҫ��
����
����
��
��2��ʵ����ϣ������뼯��ƿ��ˮ������������ݻ���
������Ϊ������һ�����ԭ�������
����������
����������
��
װ��©��
װ��©��
��
��3��ijͬѧ��ʵ����з�˼������˸Ľ���������ͼ2��ʾ ��������Ϊ�Ľ�����ŵ��ǣ�
�ð�����̫����۽���ȼ���ף���������Ƥ����ȼ������������ݳ������ʵ����ͬʱ���ɱ���������������ɢ����������Ⱦ������
�ð�����̫����۽���ȼ���ף���������Ƥ����ȼ������������ݳ������ʵ����ͬʱ���ɱ���������������ɢ����������Ⱦ������
��̫����
��ʡ��Դ������Ⱦ
��ʡ��Դ������Ⱦ
��
�������ͼʾװ�ã��ش��й����⣮
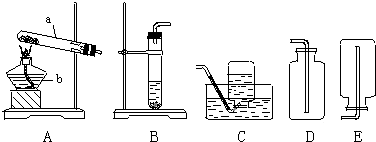
��1��д�������ָ���������ƣ�a
�Թ�
�Թ�
��b
�ƾ���
�ƾ���
����Aװ����ȡij�������һ����ѧ����ʽΪ
��
��2��ʵ�����У��ü��ȸ�����صķ�����ȡ����������װ�ÿ�ѡ��
A
A
�����ţ����������ſ������ռ�������Ӧ���������
�������ǵ�ľ�����ڼ���ƿ�ڣ��������ǵ�ľ����ȼ��˵���ռ�����
�������ǵ�ľ�����ڼ���ƿ�ڣ��������ǵ�ľ����ȼ��˵���ռ�����
��
��3��������������ȼ�ϣ������ܶȱȿ���С��������ˮ��ʵ���ҳ���п����ϡ���ᷴӦ���Ƶã��仯ѧ����ʽΪ
Zn+H2SO4�TZnSO4+H2����
Zn+H2SO4�TZnSO4+H2����
���ռ�������װ�ÿ�ѡ��
C
C
��
E
E
�������ţ�



 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
 ��1����ͼ1��ʾ���ǵ��ˮʵ��װ�ã�ͨ��һ��ʱ����������Թ��зֱ��ռ�������a������b�� ��ش�
��1����ͼ1��ʾ���ǵ��ˮʵ��װ�ã�ͨ��һ��ʱ����������Թ��зֱ��ռ�������a������b�� ��ش�