
| 106 |
| 44 |
| x |
| 2.2g |
| 142 |
| 44 |
| y |
| 2.2g |
| 80 |
| 142 |
| 4g |
| z |
| 14.2g |
| 9.3g+mg+200g-2.2g |
| 14.2g |
| 9.3g+mg+200g-2.2g |


 æŚĖ抔דŌŖæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
æŚĖ抔דŌŖæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ČēĶ¼ŹĒĪøµĆĄÖÅĘĪøŅ©µÄ²æ·Ö±źŹ¶£®ĪøŅ©ÖŠĖłŗ¬µÄĪļÖŹÖŠŗĶĪøĄļ¹ż¶ąµÄĪøĖį£®Ä³»¼Õß°“±źŹ¶ÉĻµÄ·žÓĆ·½·Ø·žÓĆŅ©ČżĢģŗó²”ĒéŗĆ×Ŗ£®
ČēĶ¼ŹĒĪøµĆĄÖÅĘĪøŅ©µÄ²æ·Ö±źŹ¶£®ĪøŅ©ÖŠĖłŗ¬µÄĪļÖŹÖŠŗĶĪøĄļ¹ż¶ąµÄĪøĖį£®Ä³»¼Õß°“±źŹ¶ÉĻµÄ·žÓĆ·½·Ø·žÓĆŅ©ČżĢģŗó²”ĒéŗĆ×Ŗ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
| A”¢¢Ł¢Ū | B”¢¢Ś¢Ū | C”¢¢Ū¢Ü | D”¢¢Ś¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Ķµ„ÖŹ | B”¢µŖĘų |
| C”¢Ņ»Ńõ»ÆĢ¼ | D”¢ĀČ»ÆÄĘČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŠņŗÅ | ĪļÖŹ | Ń”ÓĆŹŌ¼Į | ²Ł×÷ |
| A£® | Ńõ»ÆøĘ£ØĢ¼ĖįøĘ£© | Ė® | ¹żĀĖ |
| B£® | NaOHČÜŅŗ£ØNa2CO3£© | Ļ”ŃĪĖį | ¼ÓČėŹŌ¼ĮÖĮ²»ŌŁ²śÉśĘųÅŻ |
| C£® | KNO3ČÜŅŗ£ØKOH£© | Cu£ØNO3£©2ČÜŅŗ | ¼ÓČėŹŹĮæµÄŹŌ¼Į£¬Ö±½ÓÕō·¢ |
| D£® | ¶žŃõ»ÆĆĢ£ØĀČ»Æ¼Ų£© | H2O | Čܽā”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ |
| AӢA | BӢB | CӢC | DӢD |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
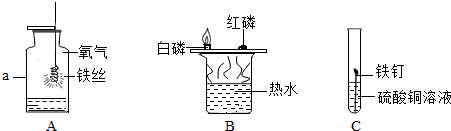
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŹµŃé·½°ø | ŹµŃéĻÖĻó | ½įĀŪ |
| ¢ŁČ”Ņ»¶ØĮæµÄŗĻ½šŃłĘ·£¬¼Ó¹żĮæµÄ ¢ŚČ”²½Öč¢ŁĖłµĆĀĖŌü£¬¼Ó |
ѳʷ²æ·ÖČܽā£¬²¢ÓŠĘųĢå·Å³ö£® |
ŗĻ½šÖŠŅ»¶Øŗ¬ÓŠ ŗĻ½šÖŠŅ»¶Øŗ¬ÓŠĢśŗĶĶ£® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŹµŃé×°ÖĆ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
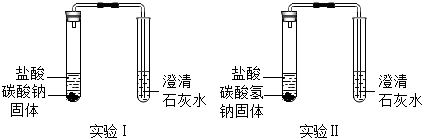 | ŹµŃé¢ń”¢¢ņÖŠ¾ł¹Ū²ģµ½£ŗ°×É«¹ĢĢåÖš½„¼õÉŁ£¬ | Na2CO3ŗĶNaHCO3¶¼ÄÜÓėŃĪĖį·“Ó¦²śÉśCO2£»NaHCO3ÓėŃĪĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ |
| t1/”ę | t2/”ę | t3/”ę | |
| Na2CO3 | 23.3 | 20.0 | 23.7 |
| NaHCO3 | 18.5 | 20.0 | 20.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com