

 ĘŚÄ©ŗĆ³É¼ØĻµĮŠ“š°ø
ĘŚÄ©ŗĆ³É¼ØĻµĮŠ“š°ø 99¼Ó1ĮģĻČĘŚÄ©ĢŲѵ¾ķĻµĮŠ“š°ø
99¼Ó1ĮģĻČĘŚÄ©ĢŲѵ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŹµŃéŠņŗÅ | ¼× | ŅŅ | ±ū |
| »ģŗĻĪļÖŹĮæ£Øg£© | 9.20 | 15.7 | 27.6 |
| Éś³ÉCO2µÄÖŹĮæ£Øg£© | 4.4 | 6.6 | 6.6 |
| £Ø¢”£© | £Ø¢¢£© | £Ø¢££© | |
| AµÄ×é³É | NaHCO3ŗĶMgCO3 NaHCO3ŗĶMgCO3 |
KHCO3ŗĶCaCO3 KHCO3ŗĶCaCO3 |
²»ŠčĢīĀś ²»ŠčĢīĀś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 £Ø2009?¹óŃō£©Ķ¼ŹĒŅ»ĘæÅØĮņĖį±źĒ©ÉĻµÄ²æ·ÖÄŚČŻ£¬Ä³Ģ½¾æŠ”×éµÄĶ¬Ń§¶ŌÕāĘæĮņĖį½ųŠŠĮĖČēĻĀĢ½¾æ£¬ĒėÄć²ĪÓė£®
£Ø2009?¹óŃō£©Ķ¼ŹĒŅ»ĘæÅØĮņĖį±źĒ©ÉĻµÄ²æ·ÖÄŚČŻ£¬Ä³Ģ½¾æŠ”×éµÄĶ¬Ń§¶ŌÕāĘæĮņĖį½ųŠŠĮĖČēĻĀĢ½¾æ£¬ĒėÄć²ĪÓė£®| ŹµŃé²½Öč | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ĻČÓĆpHŹŌÖ½²ā¶ØĻ”ĮņĖįµÄpH£¬ŌŁÖšµĪ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ²¢²»¶ĻÕńµ“£¬Ķ¬Ź±²ā»ģŗĻŅŗµÄpH | pHÖš½„±ä“ó£¬ ×īŗópH”Ż7 |
Ļ”ĮņĖįÓėĒāŃõ»ÆÄĘ ČÜŅŗÄÜ·¢Éś·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

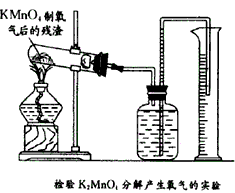
 K2MnO4+MnO2+O2”ü
K2MnO4+MnO2+O2ӟ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ķ¼ŹĒŅ»ĘæÅØĮņĖį±źĒ©ÉĻµÄ²æ·ÖÄŚČŻ£¬Ä³Ģ½¾æŠ”×éµÄĶ¬Ń§¶ŌÕāĘæĮņĖį½ųŠŠĮĖČēĻĀĢ½¾æ£¬ĒėÄć²ĪÓė£®
Ķ¼ŹĒŅ»ĘæÅØĮņĖį±źĒ©ÉĻµÄ²æ·ÖÄŚČŻ£¬Ä³Ģ½¾æŠ”×éµÄĶ¬Ń§¶ŌÕāĘæĮņĖį½ųŠŠĮĖČēĻĀĢ½¾æ£¬ĒėÄć²ĪÓė£®| ŹµŃé²½Öč | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ĻČÓĆpHŹŌÖ½²ā¶ØĻ”ĮņĖįµÄpH£¬ŌŁÖšµĪ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ²¢²»¶ĻÕńµ“£¬Ķ¬Ź±²ā»ģŗĻŅŗµÄpH | pHÖš½„±ä“ó£¬ ×īŗópH”Ż7 | Ļ”ĮņĖįÓėĒāŃõ»ÆÄĘ ČÜŅŗÄÜ·¢Éś·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com