
���й�����ȫ��ȷ��һ����
A����ѧ֮�� | B����ѧ������ |
���ܶ���С������������ �ڵؿ��к�����ߵĽ������� ����Ȼ���ڵ���Ӳ�������ǽ��ʯ | ���·��ϵ����ۣ��������ͳ�ȥ �ڱ������ζ�����û���̿���� ��ˮ���е�ˮ�����ɼ���ˮ���ݳ�ȥ |
C�����ʱ��淽�������� | D����ѧ�빤ũҵ |
����ʯ���ܷⱣ�棺��ֹ��ˮ��ʪ �����������ܷⱣ�棺��ֹ���⡢���� �۰�������ˮ�б��棺����������ֹ��ȼ | ���ö�����̼������ʯ��Ӧ���� ��ʯ�����ƿɵ����͡�ú�͡����͵� ��ϡ������ͨ����ܷ�������� |
A. A B. B C. C D. D
A ��������A���ܶ���С���������������ؿ��к�����ߵĽ���Ԫ����������Ȼ���ڵ���Ӳ�������ǽ��ʯ����A��ȷ��B��ˮ������Ҫ�ɷ���̼��ơ�������þ����Ҫ������ܳ�ȥ����B����C����ʯ��������е�ˮ��Ӧ�����������ƣ�������ʯ����Ҫ�ܷⱣ�棬��C����D��һ����̼������ʯ��Ӧ��������D����ѡA�� �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2018�긣��ʡ�����ػ�ѧ�ʼ��Ծ� ���ͣ������
��ѧ������������ء�
��1����ͼ�������г��õ�����Һ�ɷ֣�����________���ѧʽ����Cl���ϼ�Ϊ+1�ۡ�

��2����ҵ�Ͻ�������Cl2��ͨ���ռ���Һ�п���ȡ����Һ���÷�Ӧ�Ļ�ѧ����ʽΪ_____��
��3������«����ֲ���õ���������Һ�����β�������������ͭ���������ƺ�ˮ������ƶ��ɡ���������Һ�������ú�ʩ�ù����в����������Ӵ���ԭ���ǣ��û�ѧ����ʽ��ʾ��__________________��

��4�������տ����ǻƻ��˵IJ���֮һ���ʻƻ����к���һ��������ˮ�ɼ��ˮ�ɼ��������Ҵ�������ˮ�����ܡ���ˮ�����ܡ�
����ˮ�ɼ���Ҵ���Һ�У��ܼ���______________��
����ˮ�ɼ�Ļ�ѧʽΪC22H25NO6��������������Ԫ�ص���������Ϊ__________��
NaClO Cl2 + 2NaOH �� NaCl + NaClO + H2O Fe + CuSO4 = Fe SO4 + Cu �Ҵ� 3.5% ����������1�����ݡ��ڻ������У��������ϼ۵Ĵ�����Ϊ�㡱��ԭ����֪�ƵĻ��ϼ�Ϊ+1�ۣ�O�Ļ��ϼ�Ϊ-2�ۣ���NaClO�е���Ԫ��Ϊ-1�ۣ��Ȼ�������Ԫ��Ϊ-1�ۣ���2������������֪�÷�Ӧ�ķ�Ӧ�����������������ƣ����������Ȼ��ơ��������ƺ�ˮ����Ӧ��...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡ�����б�ҵ����Ӧ�Լ�⻯ѧ�Ծ� ���ͣ������
A��B��C���ֹ������ʵ��ܽ����������ͼ��ʾ����ͼ�ش����⣺
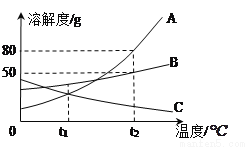
��1����t1��ʱA��B��C�������ʵı�����Һ���µ�t2�� ʱ�о����������� ________������ĸ���ţ���
��2��t2 �� ʱ����A��B���ʸ�50g�ֱ�ӵ�100gˮ�У������γɱ�����Һ���� _______������ĸ���ţ����������¶Ȳ��䣬�������������ʹ֮�ﵽ����״̬,��ʱ�ñ�����Һ��������������Ϊ_____��
C A 44.4% �����������⿼�����ܽ�����ߵ�Ӧ�ã��������������ļ��㡣 ��1��A��B���ܽ�����¶ȵ����߶�����C���ܽ�����¶ȵ����߶����٣���t1��ʱ��A��B��C�������ʵı�����Һ���µ�t2�棬�о�����������C�� ��2��t2��ʱ��A��B���ܽ�ȷֱ�80g��50g����A��B��50g�ֱ�ӵ�100gˮ�У������γɱ�����Һ����A����Ҫʹ֮�ﵽ����״̬������Ҫ����Һ�м���30gA...�鿴�𰸺ͽ���>>
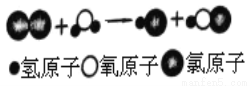
��Ŀ�����л�ѧ ��Դ������ʡ������2018����꼶�п��ڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��ͼ��ij��Ӧ����ʾ��ͼ�����з�Ӧǰ��Ԫ�ػ��ϼ�û�иı����________�����Ϸ�Ӧ���ɵ���������кͣ������Cl2ͨ��ʯ�����п���ȡƯ��[��Ч�ɷ�Ca(ClO)2��CaCl2]����ѧ����ʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2018����꼶�п��ڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ���ѡ��
��ͼΪ��ˮ����CO2������̼ѭ����ԭ��ʾ��ͼ������˵��������ǣ� ��
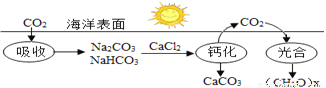
A. ����ϡ�������̫����ת��Ϊ��ѧ��
B. ���յ�CO2��ƻ����ͷŵ�CO2�������
C. �ƻ����ͷ�CO2�ķ�ӦΪ��2 NaHCO3+CaCl2==CaCO3��+CO2��+2NaCl+H2O
D. ��̼ѭ���н�CO2ת��Ϊ������л���
B ��������A����ɫֲ����еĹ�������ǽ�̫����ת��Ϊ��ѧ�ܵĹ��̣�����ȷ��B�����յ�CO2��ƻ����ͷŵ�CO2��������ȣ����յĶ�����̼�࣬�ʴ���C���ƻ����ͷ�CO2�ķ�ӦΪ��2NaHCO3+CaCl2=CaCO3��+CO2��+2NaCl+H2O������ȷ��D��̼ѭ���н�CO2����ˮ����ת��Ϊ̼�ᣬΪ�����ɫֲ����еĹ�����ã���������̼ת��Ϊ�л������ȷ����ѡB���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2018����꼶�п��ڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ���ѡ��
����ˮ��ɽ���ǽ�ɽ��ɽ�������������Ȼ��г����������ʱ���ҹ���չ�Ļ�������֮һ������������ȷ���ǣ� ��
A. �͵�ȼ�սոѣ������������� B. ������ɽ������ũ����ƶ
C. ������ˣ���չ��ɫ������� D. ����ʹ�û��ʣ�ֲ�������̻���ɽ
C ��������A���͵�ȼ�սոѻ���������к�����ͷ۳�����Ⱦ�������ʴ���B��������ɽ���������Դ�˷ѣ��ʴ���C��������ˣ���չ��ɫ������Σ����Ա�������������ȷ��D������ʹ�û��ʡ�ũҩ�����������ջ���Ⱦ�������ʴ���ѡC���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�ܿ���2018����꼶��ѧ����ĩѧҵ���Ի�ѧ�Ծ� ���ͣ������
��1������һƿ���治�õ����������ƹ��壬����֤�����Ƿ���ʣ���д��ѡ��(���ֲ�ͬ��������)�Ļ�ѧ����ʽ: ________________��_____________��
��2�������������Ƶ���ҺA.30% 1ml��B.20% 5ml C.10% 10ml��D.1% 20ml����ô������Һ������pH��С�����˳��Ϊ______��
Na2CO3 + 2HCl =2NaCl + H2O + CO2�� Ca(OH)2 + Na2CO3 = CaCO3��+ 2NaOH ( ��CaCl2 + Na2CO3 = CaCO3��+ 2NaCl������������) D��C��B��A ����������1�������������ƵĻ�ѧ���ʽ��з���������������������еĶ�����̼��Ӧ����̼���ƺ�ˮ���������Ʊ��ʺ����ɵ�������̼���ƣ���˼����Ƿ���ʼ������Ƿ��...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ̩����2018����꼶�п���ģ��ѧ�Ծ� ���ͣ��ۺ���
������Ҫ�ɵ��ǡ�����Ĥ�����ס����Ƶȼ���������ɣ����е��ǵ���Ҫ�ɷ���CaCO3�����渲��һ�㵰��Ĥ����ѧ���ȤС���ͬѧ��չ������̽�����
��������⣩�����е�CaCO3�����Ƕ��٣�
���������ϣ�
a�����Ǿ�����ѧ������к�CaCO3����������ԼΪ82%~85%������������������Լ14-17%��
b������Ĥ�е����ʺ���ԼΪ90%~93%��
����Ʒ�����
����һ���á����շ��������õ��Ƿ��ڿ����г�����ղ��ɼ�������ݽ��вⶨ��
���������á��ᴦ����������������ϡ���ᷴӦ���в���������
������ʵ�飩
����һ����ȡ12.0 g������ĥ�ɷ�ĩ����ͨ����У��������������ټ��٣��ٳ���ʣ����������Ϊ7.16 g��
������������ͼ��ʾ��ʵ��װ�ã���ȡ12 g������Ʒ����ʵ��(���������ɷ־�����HCl��Ӧ��װ�����Լ���������)
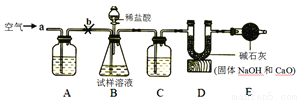
��ʵ�鲽�裩
�� ����ͼ���Ӻ�װ�ú����װ�õ������ԣ�
����Bװ���м��뵰����Ʒ����ͨ��һ���������
�۳���װ��D������ҩƷ������������Ʒ�е���5%��ϡ���ᣬֱ��________Ϊֹ��
���ٻ���ͨ�����һ�������װ��D��������������Ϊֹ���ݳ���װ��D������ҩƷ��������
�����ݴ�����
����һ����С��ͬѧ��Ϊ�������Ϊ�ֽ�����Ķ�����̼�������ݴ����������CaCO3����������Ϊ91.7%
����������÷�Ӧ��Dװ�ü�����ҩƷ��������������4.4g����Ʒ��̼��Ƶ����������Ƕ���________�� (д��������̣���������ȷ��0.1%)
�����۷�˼��
(1)����һ�������ֽⷴӦ�Ļ�ѧ����ʽΪ________���ⶨֵ���ѧ���������нϴ�����Ҫԭ����________��
(2)��������װ��A�еĻ�ѧ����ʽΪ________,��װ��C�е�ҩƷΪŨ���ᣬ��������________,װ��E��������________��
���ٲ������� 83.3% CaCO3CaO��CO2�� ���Ǽ�����Ĥ�е��л�������Ҳ����������̼�����嵼�²ⶨ���ƫ�� CO2 +2NaOH= Na2CO3��H2O �������壬��ȥˮ���� ��ֹ�����еĶ�����̼��ˮ��������װ��D��Ӱ��ⶨ��� ������������ͨ��ʵ��̽�����ʵ���ɳɷֿ����˳�������ļ�������ӷ������εĻ�ѧ���ʣ����ݻ�ѧ����ʽ���㡣 ʵ�鲽�裺��ȷ����������̼��������ʵ���...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������2018����꼶��ѧ�ڵڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ�������
þͭ�Ͻ������ɻ����ߵȵ�����ϣ���ȡijþͭ�Ͻ�3g�����ձ��У�����50gϡ����ǡ����ȫ��Ӧ����Ӧ�����ձ���ʣ���������Ϊ52.8g������㣺
(1)����H2������Ϊ____________��
(2)��ϡ���������ʵ���������Ϊ____________��
0.2g 19.6% �����������⿼����Ǹ��ݻ�ѧ����ʽ�ļ��㣬�����Ĺؼ����ձ��е����ʼ��������������ɵ����������� ��1�����������֪������H2������=50g+3g-52.8g=0.2g�� ��2��þͭ�Ͻ����ϡ�����У�ֻ��þ��Ӧ���裺����0.2g������Ҫ���������Ϊx�� Mg+H2SO4�TMgSO4+H2�� 98 2 x 0.2g x=9.8g�� ...�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com