
��3�֣���������ƣ�Na2S2O3����һ����;�㷺�����ʡ�ij�����������Ʒ�к��������������ơ���ȡ16 g����Ʒ�����ձ��У�����113.6 gһ����������������ϡ����ǡ����ȫ��Ӧ���õ�120 g�����Ʋ�������Һ��
������Ӧ�Ļ�ѧ����ʽΪ��Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2��
����㣺
��1����Ʒ����������ƣ�Na2S2O3���������Ƶ������ȡ�
��2��������Һ����������������
��1��79��1 ��2��12%
���������������1�����������غ㶨�ɿ����жϣ�����S��SO2��������=16g+113.6g-120g=9.6g���ٸ��ݻ�ѧ����ʽ��Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2����S��SO2��������=1��2������S������Ϊx����SO2������Ϊ2x������X+2x=9.6g��x=3.2g
�⣺ ���������������Ϊy�����ɵ�����������Ϊz
Na2S2O3 + H2SO4=== Na2SO4 + H2O + S��+ SO2��
158 142 32 64
y z 3.2 g
y="15.8" g z="14.2" g
��1����Ʒ������������������Ƶ�������Ϊ��15.8 g : (16 g - 15.8 g) = 79:1
��2����������Һ��������������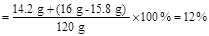
�𣺣�1����Ʒ������������������Ƶ�������Ϊ79:1��
��2����������Һ��������������Ϊ12%��
���㣺���ݻ�ѧ����ʽ���еļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣�ijͬѧ��������ʵ�飺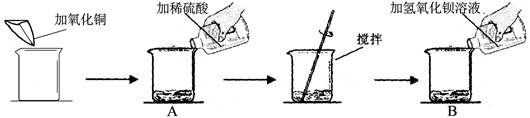
ʵ�����ݼ�����ʵ���������±���
| | ��һ��[��Դ:ѧ���ƣ���Z��X��X��K] | �ڶ��� |
| ������ͭ��������g�� | m | m |
| ��ϡ�����������g�� | 50 | 100 |
| ������������Һ��������g�� | 100 | 100 |
| B����Ҫ���� | ����ɫ���� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��9�֣�ʵ������һƿ������Һ����ʦ��С��ͬѧ��Ʒ����ⶨ�÷�Һ�����������������С��ͬѧ��ȡһ�ྻС�ձ�����������Ϊ18.2g��Ȼ�������е������������Һ�������������Ϊ33.2g��֮��һö����Ϊ10.8g������������ɰֽ��ĥȥ�����⣩�����С�ձ��з�Ӧ�����������治�������ݲ������ٴγ�����������Ϊ43.9 g��
��ش��������⣺
��1����Ӧ�в���������������� ��
��2������÷�Һ�����������������д��������̣�����������һλС��������6�֣�
��3���������������δ�������Լ�������Ӱ���� (ѡ�ƫ����ƫС��������Ӱ�족)��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��8�֣�
ij��ѧ��ȤС��ʹ����ͼ��ʾװ�ã���ij��пͭ�Ͻ�ijɷֽ��в�������ȡ����ϡ�������ձ��У��������м���15��0g�Ͻ���Ʒ��ʼ��ʱ������������ƽ�Ķ�����¼���±��У�������м��㣺
��1����Ӧ��ȫ����������������Ƕ��٣�
��2��пͭ�Ͻ���ͭ�������Ƕ��٣�
��3����Ӧ��ȫ����Һ�����ʵ����������Ƕ��٣�
| | ���ձ� | ��������� | ����Ͻ�� 5���� | ����Ͻ�� 10���� | ����Ͻ�� 30���� |
| ������g�� | 21��3 | 169��7 | 184��6 | 184��3 | 184��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(4��)ijУ��ѧ��ȤС���ͬѧΪ����ȡ������þ������ȡ9.5g�Ȼ�þ����ȫ���ܽ���40.5gˮ���Ƴɲ�������Һ��Ȼ�������м���55.8gij������������������������Һǡ����ȫ��Ӧ������㣺
(1)�Ƶõ�������þ��������
(2)��Ӧ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��8�֣�ijͬѧ��ȡa gͭп�Ͻ𣬼�������ϡ���ᣬ��ַ�Ӧ�����������������Ϊb g��ʣ����徭���ˡ�ϴ�ӡ�����Ƶ�����Ϊc g�����ʣ�
��1��a gͭп�Ͻ���п������Ϊ����g���ô���ʽ��ʾ����
��2�����ݻ�ѧ��Ӧ����ʽ���Ƶ���a��b��c����֮��ĵ�����ϵ��д����ϸ�ļ����Ƶ����̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��12�֣�ˮ������֮Դ������Ȼ������Ҫ�����ʡ�
����Ȫ�����������ǿ������ȵ�һ����ʽ���Ͼ���ɽ��Ȫ�����ƾõ��Ļ���
��1���峺����Ȫˮ�� ����������������
��2��������Ȫˮ�Ĺ����У���ʹ�õ�����̿����Ҫ�������� ���á�
��3��������Ȫˮ��Ӳˮ������ˮ�������Լ��� ��
��ˮ�Ǿ����Դ���⡣��ˮ��ɹ�ɵõ����Σ���֪ij������Ʒ�г���ɳ�����Na2SO4��MgCl2��CaCl2�����ʡ�ʵ�����ᴿ�������£� 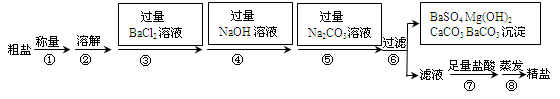
��1����������ƽ����10.2g������Ʒʱ����ָ��ƫ����ߣ����ʾ������ĸ��ţ� ��
| A�������أ������� | B�������ᣬ��Ʒ�� |
| C�������أ���Ʒ�� | D�������ᣬ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ͭ��ͭ��п�ĺϽ𣬿�����������������������������Ʒ��Ϊ�˲ⶨij��ͭ��Ʒ��ͭ������������ȡ20g����Ʒ�����м���һ������ϡ���ᣬǡ����ȫ��Ӧ���������������������ϡ�����������ϵ��ͼ��ʾ��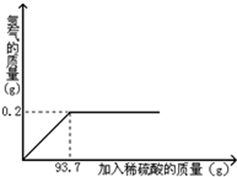
��1����ȫ��Ӧ������H2������Ϊ g��
��2����ȫ��Ӧ��������Һ���������������Ƕ���
������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
100g8%��NaOH��Һ��ǡ������100gϡ������ȫ��Ӧ�����ϡ���������ʵ����������Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com