
 С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ������Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶ�����������ȡ16.25g������Ʒ��ȫ������143.6gˮ�У������õ��Ļ����Һ����μ���������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ��
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ������Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶ�����������ȡ16.25g������Ʒ��ȫ������143.6gˮ�У������õ��Ļ����Һ����μ���������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ������ ����ͼ���֪�����ߵ��۵�����Ӧ��̼������Һ��������ǡ����ȫ��Ӧ����������̼������Һ�����������ݷ�Ӧ�Ļ�ѧ����ʽ������ǡ����ȫ��Ӧʱ����̼���Ƶ����������ɳ�����������������̼���Ʒ�����Ӧ���Ȼ�����������ǡ����ȫ��Ӧ����ˣ�������ҺΪ�Ȼ�����Һ�������Ȼ��Ƶ�����Ϊ��Ʒ���Ȼ����뷴Ӧ�����Ȼ��������ͣ���Һ�����������������غ㶨�ɽ��м��㣻�ɷ�Ӧ����������Ȼ��Ƶ��������������������������㹫ʽ���������Һ�������Ȼ��Ƶ�����������
��� �⣺��1���������߿�֪��������̼������Һ50gʱ��ǡ����ȫ��Ӧ��
��2������Ʒ��BaCl2������Ϊx������NaCl������Ϊy
BaCl2+Na2CO3�TBaCO3��+2NaCl
208 106 117
x 50g��10.6% y
$\frac{208}{x}$=$\frac{106}{50g��10.6%}$=$\frac{117}{y}$
x=10.4g
y=5.85g
����ԭ�������Ȼ��Ƶ�������16.25g-10.4g=5.85g
��Ʒ���Ȼ��Ƶ����������ǣ�$\frac{5.85g}{16.25g}$��100%=35.9%
��3��ˮ���ܶ�Ϊ1g/cm3����143.6mLˮ������Ϊ143.6mL��1g/cm3=143.6g��
���Ȼ�����̼����ǡ����ȫ��Ӧʱ�����˺�������Һ������Ϊ��16.25g+143.6g+50g-9.85g=200g��
����Һ�����ʵ���������Ϊ��$\frac{5.85g��2}{200g}$��l00%=5.85%��
�ʴ�Ϊ����1��50��
��2��35.9%��
��3��5.85%��
���� �����Ĺؼ���ץס���ڶԷ�Ӧͼ�������߽��з���ʱ�����ߵ��۵����ζ����������ʾ�ķ�Ӧ�ڴ�ʱǡ����ȫ��Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
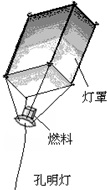 Ԫ�������ҹ�����Ҫ��ͳ���գ�ȼ�ſ����ƣ���ͼ����������Ԫ���ڵ��·��У�ij�н�ֹȼ�ſ����ƣ���Ϊ������ȼ��ʱ�����¶ȸߴ�300��ȣ�һ���䵽����վ��Һ����վ���Ϳ�ȵأ������ƻ�ʹ�¶ȴﵽ��ȼ����Ż���������֣�
Ԫ�������ҹ�����Ҫ��ͳ���գ�ȼ�ſ����ƣ���ͼ����������Ԫ���ڵ��·��У�ij�н�ֹȼ�ſ����ƣ���Ϊ������ȼ��ʱ�����¶ȸߴ�300��ȣ�һ���䵽����վ��Һ����վ���Ϳ�ȵأ������ƻ�ʹ�¶ȴﵽ��ȼ����Ż���������֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��K2SO4��MgCl2 | B�� | ZnSO4��MgSO4��CuSO4 | ||
| C�� | NaCl��K2SO4��Mg��NO3��2 | D�� | NaCl��FeCl3��KCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼��ˮ�����������Ŀ���ǽ��Ϳ�ȼ����Ż�� | |
| B�� | ��ú�Ƴɷ���ú����ȼ����Ϊ��ʹ��ȼ�ո��ӳ�� | |
| C�� | ����ȼ��ʱ��Ӧ��һ���������μ� | |
| D�� | ú����Ȼ����ʯ�ͳ���Ϊ��ʯȼ�ϣ����ǿ�������Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
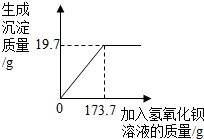 Ϊ�ⶨ�������ƹ�����Ʒ�ı��ʳ̶ȣ���ѧ��ȤС���ͬѧ����������ʵ�飺��ȡ20.0g������Ʒ�����ձ��У���������������Һ�����ٲ�������Ϊֹ�����˺õ�һ����������Һ�ͳ����������Ƽ�������������Һ��������������Ĺ�ϵ��ͼ��ʾ�������������⣺
Ϊ�ⶨ�������ƹ�����Ʒ�ı��ʳ̶ȣ���ѧ��ȤС���ͬѧ����������ʵ�飺��ȡ20.0g������Ʒ�����ձ��У���������������Һ�����ٲ�������Ϊֹ�����˺õ�һ����������Һ�ͳ����������Ƽ�������������Һ��������������Ĺ�ϵ��ͼ��ʾ�������������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com