


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)ijУ�����뻯ѧ���С��ͨ�����������˽���ҹ�����ÿ������Լ40������ϴ����ٶ���Щʹ�ú�ķ����ϴ���ͨ�����մ��������ŷŴ���CO2���ûС������ͼ��ʾװ�ã�����һ���������ϴ�ȼ�պ������CO2��������
(1)Ϊ�˱�֤������ȷ�ԣ����Ӻ�ʵ��װ�ú�Ӧ���________________________���ټ���ҩƷ����ʵ�顣
(2)װ�â��С�ձ��м�������H2O2�ʹ������ɳ�������O2�����ϴ����ȼ�գ�����O2�Ļ�ѧ����ʽ��__________________________��
(3)���ϴ�ȼ����װ�â�����ȴʱ��װ�â���Һ��Ҳ����������װ�â��У�ԭ����____
_______________
(4)����ʵ��Ŀ�ģ���Ҫ��������_________��
A��ʵ��ǰ���ϴ�������
B��ʵ��ǰ��ʵ���װ�â������
C.ʵ��ǰ��ʵ���װ�â������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ��㶫���ݾ�����ѧ ���ͣ������
(8��)ijУ�����뻯ѧ���С��ͨ�����������˽���ҹ�����ÿ������Լ40������ϴ����ٶ���Щʹ�ú�ķ����ϴ���ͨ�����մ��������ŷŴ���CO2���ûС������ͼ��ʾװ�ã�����һ���������ϴ�ȼ�պ������CO2��������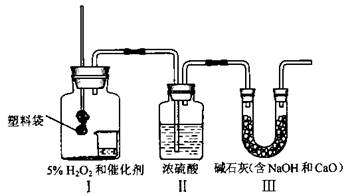
(1)Ϊ�˱�֤������ȷ�ԣ����Ӻ�ʵ��װ�ú�Ӧ���________________________���ټ���ҩƷ����ʵ�顣
(2)װ�â��С�ձ��м�������H2O2�ʹ������ɳ�������O2�����ϴ����ȼ�գ�����O2�Ļ�ѧ����ʽ��__________________________��
(3)���ϴ�ȼ����װ�â�����ȴʱ��װ�â���Һ��Ҳ����������װ�â��У�ԭ����____
_______________
(4)����ʵ��Ŀ�ģ���Ҫ��������_________��
A��ʵ��ǰ���ϴ�������
B��ʵ��ǰ��ʵ���װ�â������
C.ʵ��ǰ��ʵ���װ�â������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ��㶫���ݾ�����ѧ ���ͣ������
(8��)ijУ�����뻯ѧ���С��ͨ�����������˽���ҹ�����ÿ������Լ40������ϴ����ٶ���Щʹ�ú�ķ����ϴ���ͨ�����մ��������ŷŴ���CO2���ûС������ͼ��ʾװ�ã�����һ���������ϴ�ȼ�պ������CO2��������

(1)Ϊ�˱�֤������ȷ�ԣ����Ӻ�ʵ��װ�ú�Ӧ���________________________���ټ���ҩƷ����ʵ�顣
(2)װ�â��С�ձ��м�������H2O2�ʹ������ɳ�������O2�����ϴ����ȼ�գ�����O2�Ļ�ѧ����ʽ��__________________________��
(3)���ϴ�ȼ����װ�â�����ȴʱ��װ�â���Һ��Ҳ����������װ�â��У�ԭ����____
_______________
(4)����ʵ��Ŀ�ģ���Ҫ��������_________��
A��ʵ��ǰ���ϴ�������
B��ʵ��ǰ��ʵ���װ�â������
C.ʵ��ǰ��ʵ���װ�â������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com