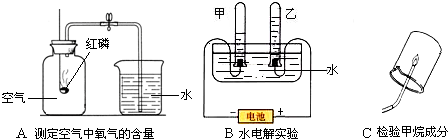
������ͼ�dz��л�ѧѧϰ��������̽��������ɵ�����ʵ�飺
��1��Aʵ�������ÿ�ȼ������ƿ�ڵ�______�����ѧʽ��ʹƿ��ѹǿ��С���Ʋ���������������ģ�����ľ̿������ף��ܲ��ܴﵽʵ��Ŀ�ģ�______��
��2��Bʵ����ͨ��֤��������ijɷ����ƶ�ˮ����ɵģ��ڼס�����֧�Թ����ռ���������ֱ���______�����ǵ������Ϊ______��
��3��Cʵ����A��Bʵ���е�______����ʵ����ţ�ʵ��ķ�����ͬ��д��������ȫȼ�յĻ�ѧ����ʽ______ 2H2O+CO2
���𰸡�
����������ͨ���Բⶨ����������������ʵ�顢���ˮʵ�顢����ȼ��ʵ���ԭ�����з������ó�����Ĵ𰸣�
����⣺��1���ⶨ����������������ʵ��ԭ���ǣ�����ȼ�գ����ļ���ƿ�е��������Ӷ�ʹ����ƿ�������ѹǿ��ձ��е�ˮ��ѹǿ������������뼯��ƿ�У��̶��ⶨ�����뼯��ƿ��ˮ�����������ˮ������뼯��ƿ���ݻ���ȣ����ܵõ�ʵ��Ľ��ۣ������̼��ȼ���������������̼�������γ�ѹǿ�
�ʴ�Ϊ��O
2�� ����
��2�����ˮʵ���ԭ���ǣ�ˮͨ��ֽ���������壬ͨ��ʵ������ȷ���������������������������������غ㶨�ɿ�֪ˮ�к����⡢������Ԫ�أ�ͨ��������ܶȺ���������Լ������������������ȣ����������غ㶨�ɿ�֪���������Ⱦ���ˮ���⡢��Ԫ�ص������ȣ�ͨ���������ȿ��Եõ�ˮ����ɺ��ɣ�
�ʴ�Ϊ����������������H
2�� O
2���� 2��1
��3������ȼ�յ������ǣ�������ɫ���棬���ȣ��ձ�������ˮ����֣�����ʹ����ʯ��ˮ����ǵ����壮�ɡ��ձ�������ˮ����֡�˵��������ˮ��ˮ���⡢��Ԫ����ɣ����������غ㶨�ɿ�֪������һ��������Ԫ�أ��ɡ�����ʹ����ʯ��ˮ����ǵ����塱˵�������˶�����̼��������̼��̼����Ԫ����ɣ����������غ㶨�ɿ�֪������һ������̼Ԫ�أ�ͨ��ʵ��ⶨ��ˮ�Ͷ�����̼������������ȷ���������Ƿ�����Ԫ�أ�
ͨ��������������֪��Aʵ�������ÿ�ȼ������ƿ�ڵ�������ʹƿ��ѹǿ��С���Ʋ���������������ģ�Bʵ����ͨ��֤���������Ԫ�����ࣨ����ɡ��ɷ֡������ƶ�ˮ����ɵģ���Cʵ����Bʵ��ķ�����ͬ��
�ʴ�Ϊ��B�� CH
4+2O
2
2H
2O+CO
2������������Ҫ����ⶨ����������������ʵ�顢���ˮʵ�顢����ȼ��ʵ���ԭ�����Ѷ��Դ�
��Ŀ�����л�ѧ
��Դ��2011-2012ѧ������ʡ��ͨ��ˮ�����о��꼶���£����л�ѧ�Ծ��������棩
���ͣ������
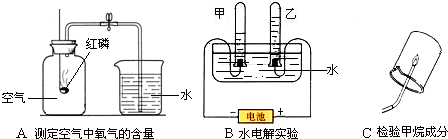
������ͼ�dz��л�ѧѧϰ��������̽��������ɵ�����ʵ�飺
��1��Aʵ�������ÿ�ȼ������ƿ�ڵ�______�����ѧʽ��ʹƿ��ѹǿ��С���Ʋ���������������ģ�����ľ̿������ף��ܲ��ܴﵽʵ��Ŀ�ģ�______��
��2��Bʵ����ͨ��֤��������ijɷ����ƶ�ˮ����ɵģ��ڼס�����֧�Թ����ռ���������ֱ���______�����ǵ������Ϊ______��
��3��Cʵ����A��Bʵ���е�______����ʵ����ţ�ʵ��ķ�����ͬ��д��������ȫȼ�յĻ�ѧ����ʽ______ 2H2O+CO2
�鿴�𰸺ͽ���>>

 2H2O+CO2
2H2O+CO2