��2005?������һģ��2004��6��5�������绷���գ��������ǡ����������ƥ�����𡱣���������ά�ֺ���Ŀɳ�����չ��ÿ�����������ʥְ����ش�������ں���ļ������⣺
[����һ]ȥ��5�£��ҹ��㽭�غ�����������ġ��ೱ���������ֳҵ�ش���ʧ���ೱ�ķ�����Ҫ������Ϊ����ķ��������������賤��������ص�Ԫ����
N��P
N��P
����Ԫ�ط��ţ�
[�����]��ˮ����Դ�ḻ���������Ľ�����������
Na+
Na+
�������ӷ��ţ����������ķ�����
H2O
H2O
���ѧʽ����
[������]�ҹ���ɽ�γ���ˮɹ�ε�ԭ����
B
B
��������ţ�
A���Ȼ��Ƶ��ܽ�����¶�Ӱ��ϴ�
B�����Ȼ����ܽ�����¶�Ӱ�첻�ʲ�ȡ����ˮ�ֵķ�����
���ô�������Ҫ����ɰ���Ȼ�þ���Ȼ��Ƶ����ʣ��ֶ�������ᴿ����Ҫ�����������£�

��1�������ٵ�������
����
����
�������ڵ�������
����
����
��
��2��A��Һ��
a
a
��B��Һ��
d
d
����ѡ����������գ�
a��Na
2CO
3 b��K
2CO
3 c��H
2SO
4 d��HCl
[������]�����죬��º��зḻ�ġ���ȼ����������������1000�����Դ��Ҫ����������һ�־��壬������ƽ��ÿ46��ˮ���ӹ�����8������ÿ����������1��CH
4���ӻ�1������H
2O���ӣ���������ÿ8����ֻ��6��������CH
4���ӣ�����2����������H
2O������䣬����Ȼ��ˮ�����ƽ����ɿɱ�ʾΪ
B
B
��
A��CH
4?14H
2O B��CH
4?8H
2O C��CH
4?��23/3��H
2O D��CH
4?6H
2O
����ˮ����ɱ�ʾΪCH
4?nH
2O��������������ȫȼ�յķ���ʽ���£��뽫�����еĻ�ѧ����������������
CH
4?nH
2O+2O
2CO
2+
n+2
n+2
H
2O��
[������]��ˮ�����ǹ������о����ȵ����⣬�ҹ���ѧ�������ø߷���Ĥ���к�ˮ�������о���ȡ����һЩ�ɼ�����ͼ�����Ϊ��ˮ���Ҳ�Ϊ����һ��ʱ�������ྭ�߷���Ĥ���õ��ĵ�ˮ�������߷���ĤӦ���е�������
ʹˮ����ͨ�������ӣ������ӡ�þ���ӵȣ�����ͨ��
ʹˮ����ͨ�������ӣ������ӡ�þ���ӵȣ�����ͨ��
��
[������]��ͼΪ������������һ�ּ��û���������Һ��������ԭ���ǵ�ⱥ���Ȼ�����Һ���Ƶõ�����Һ�н�ǿ��ɱ���������÷�Ӧ�������Ȼ��ƺ�ˮ�ڵ������������NaOH��H
2��Cl
2������ɻ�ѧ����ʽ��
2NaCl+2H
2O
2NaOH+H
2��+Cl
2��
2NaCl+2H
2O
2NaOH+H
2��+Cl
2��
��
������Һ�����������õ�������NaClO���������ƣ���������������NaOH��һ����Ӧ�Ƶõģ���Ӧ����ʽΪ��2NaOH+Cl
2=NaCl+NaClO+H
2O����ģ��д��Cl
2����ʯ��������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ��
2Cl2+2Ca��OH��2=Ca��ClO��2+CaCl2+2H2O
2Cl2+2Ca��OH��2=Ca��ClO��2+CaCl2+2H2O
��
2005��3��29�գ�����ʡ��������������Cl
2��й©���ش��¹ʣ��¹ʷ��������ն�Ա������ɢ����Ⱥ�ڣ�Ѹ�ٽ�����й©������������ˮ�У������������Գ�������Ļ���ɫ���壨��������ˮ��������Һ����ֹ�����Ի����Ľ�һ����Ⱦ�Ͷ���Ա���˺����Խ��������Ի�������Ⱦ��
��1����������Ϣ�У����ܹ�������������ѧ����Ϊ��
��Ӧ����ˮ��Ӧ
��Ӧ����ˮ��Ӧ
��
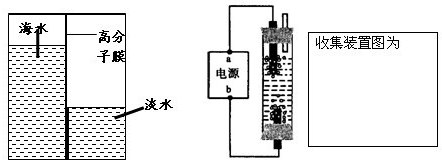
��2�����ʵ������ȡ�������������ʲô�����ռ����������ռ�װ��ͼ��
��3���������뿪�ֳ��Ĺ����У���Ӧ�ò�ȡʲô��ʩ�����Լ���



 ��������ϵ�д�
��������ϵ�д�






 ��������ĸ��ʾ���dz��л�ѧ���������ʣ�������H��C��O��Na��S��Ca��Fe�еļ���Ԫ����ɣ�
��������ĸ��ʾ���dz��л�ѧ���������ʣ�������H��C��O��Na��S��Ca��Fe�еļ���Ԫ����ɣ�