



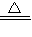 2CuO����CuO+H2SO4�TCuSO4+H2O����Fe+CuSO4�TFeSO4+Cu���������ա��������Ⱦ���������塢�۳������������Բ����ҷ�����������Ȼ�������ȡ���й����⣻���ѧ���ļ���������������ճɹ�����ǿ�ɾУ�
2CuO����CuO+H2SO4�TCuSO4+H2O����Fe+CuSO4�TFeSO4+Cu���������ա��������Ⱦ���������塢�۳������������Բ����ҷ�����������Ȼ�������ȡ���й����⣻���ѧ���ļ���������������ճɹ�����ǿ�ɾУ�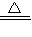 2CuO��
2CuO��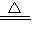 2CuO+2SO2��+O2����
2CuO+2SO2��+O2����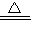 CuSO4+xH2O����
CuSO4+xH2O����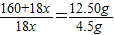
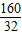 =5��
=5�� ×100%=64%
×100%=64% 2Cu2O+O2����
2Cu2O+O2���� 2CuO����CuO+H2SO4=CuSO4+H2O����Cu+H2O2+H2SO4=CuSO4+2H2O��
2CuO����CuO+H2SO4=CuSO4+H2O����Cu+H2O2+H2SO4=CuSO4+2H2O�� 2CuO+2SO2��+O2��
2CuO+2SO2��+O2�� 2Cu2O+O2��
2Cu2O+O2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
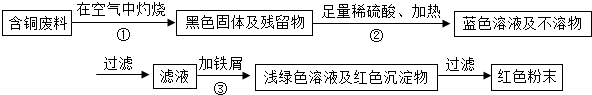
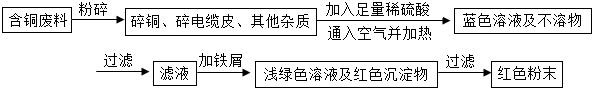
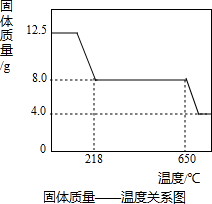 ij��ҵ��һ���������������ƷΪ���ĵط�������ҵ���ڵ������������У����ɱ���ػ����һ�����ĺ�ͭ���ϣ��磺������£���ij��ѧ��ȤС���ͬѧ��֪��һ�������������ú�ͭ�����Ʊ�������CuSO4?5H2O����
ij��ҵ��һ���������������ƷΪ���ĵط�������ҵ���ڵ������������У����ɱ���ػ����һ�����ĺ�ͭ���ϣ��磺������£���ij��ѧ��ȤС���ͬѧ��֪��һ�������������ú�ͭ�����Ʊ�������CuSO4?5H2O����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ������



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ�������п���ѧ��ģ�Ծ��������棩 ���ͣ������


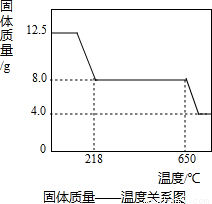
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com