

 2CuO��H2+CuO
2CuO��H2+CuO Cu+H2O
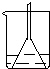
Cu+H2O Cu+H2O֪�������ܶ�ȡ����ͭ������������ǻ�ԭ�����ʴ�Ϊ����ԭ��
Cu+H2O֪�������ܶ�ȡ����ͭ������������ǻ�ԭ�����ʴ�Ϊ����ԭ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ɺ�ɫ��ع����ĺ�ɫ��
�ɺ�ɫ��ع����ĺ�ɫ��
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ۺ�Ӧ�ô������¿α�9�꼶��ѧ�� ���ͣ�013
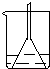
ijѧ���ڼ����������µĻ�ѧʵ�飺һ���������ᷴӦ��С�����ʢ��������ձ��У�С��Ư����Һ��(λ����ͼ��ʾ)��Ȼ�ӷϾɵ������ռ�����пƬ(����)Ͷ���ձ��У������������ݲ���Ϊֹ(��Һ����仯���Բ���)���뿪ʼʱ��ȣ�С��������λ�ý�
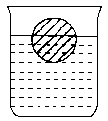
[����]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ɺ�ɫ��ع����ĺ�ɫ��
�ɺ�ɫ��ع����ĺ�ɫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ˮ�� ���ͣ��ʴ���
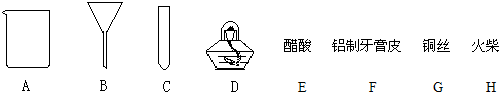
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com