
 ×Ö“Ź¾äĘŖÓėĶ¬²½×÷ĪÄ“ļ±źĻµĮŠ“š°ø
×Ö“Ź¾äĘŖÓėĶ¬²½×÷ĪÄ“ļ±źĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A | B | C | D | E | |
A |
”ż | ”ż | ”ż | ©„ | |
| B | ”ż | ”ü | ©„ | ©„ | |
| C | ”ż | ”ü | ©„ | ©„ | |
| D | ”ż | ©„ | ©„ | ©„ | |
| E | ©„ | ©„ | ©„ | ©„ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
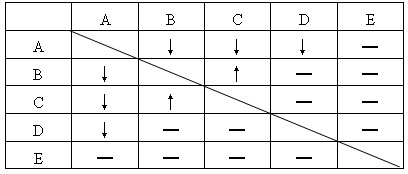
ĻÖÓŠNaNO3”¢AgNO3”¢HCl”¢Na2CO3”¢NaCl”¢Ca(NO3)2ĮłÖÖČÜŅŗ£¬½öCa(NO3)2ČÜŅŗĘæÉĻĢłÓŠ±źĒ©”£ĪŖČ·¶ØĘäĖüĪåÖÖČÜŅŗø÷ŹĒŹ²Ć“£¬½«ĖüĆĒĖęŅā±ąŗÅĪŖA”¢B”¢C”¢D”¢Eŗó£¬Į½Į½»ģŗĶ½ųŠŠŹµŃ飬øł¾ŻŹµŃéĻÖĻó(ČēĻĀ±ķĖłŹ¾)»Ų“š£ŗ
| A | B | C | D | E | |
| A | ”ż | ”ż | ”ż | ©„ | |
| B | ”ż | ”ü | ©„ | ©„ | |
| C | ”ż | ”ü | ©„ | ©„ | |
| D | ”ż | ©„ | ©„ | ©„ | |
| E | ©„ | ©„ | ©„ | ©„ |
(1)°ŃÓÉŹµŃéĻÖĻóÄÜČ·¶ØµÄA”¢D”¢EµÄ»ÆѧŹ½ĢīŌŚĻĀ±ķĻąÓ¦µÄæÕøńÖŠ”£
A D E
(2)ĪŖĮĖČ·¶ØB”¢CĪļÖŹø÷ŹĒŹ²Ć“£¬»¹ŅŖ½ųŠŠČēĻĀŹµŃ锣ĢīæÕ»Ų“šÓŠ¹ŲĪŹĢā£ŗ
¢Ł“ÓŅŃČ·¶ØµÄŹŌ¼ĮÖŠŃ”ÓĆ____________×÷ĪŖ¼ų±šŹŌ¼Į×ī¼ņµ„”£
¢ŚĻņĪ“Č·¶ØµÄB”¢CČÜŅŗÖŠ·Ö±š¼ÓČė¢ŁÖŠŃ”ÓĆµÄ¼ų±šŹŌ¼Į£¬ÓŠ°×É«³ĮµķÉś³ÉµÄŌČÜŅŗ»ÆѧŹ½ŹĒ__________ĪŽ·“Ó¦ĻÖĻóµÄŌČÜŅŗ»ÆѧŹ½ŹĒ__________”£
¢ŪA+D·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com