
 2H2O+O2����
2H2O+O2���� 2H2O+O2��
2H2O+O2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ʵ��װ�� | ʵ����� | ʵ�������ͻ���� |
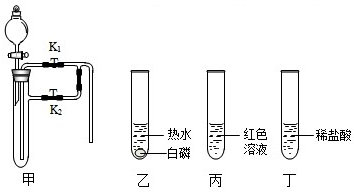
��1�� |
 |
�ٹرջ���K����ľ̿���ȣ� ��Ϩ��ƾ��ƣ���ľ̿��ȴ����K��ͨ�������� �۵�ȼ�ƾ��ƣ���ľ̿���ȣ� |
��ľ̿��ȼ�գ� ��ľ̿��ȼ�գ� ��ľ̿ȼ�գ� �ɴ�˵����ȼ��ȼ�յ������� �������Ӵ����¶ȴﵽ�Ż�㣮 �� |
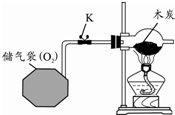
��2�� |
 |
��Һ©���IJ������ͻ���K����ϡ����ע��С�Թ��У� |
�۲쵽�������� С�Թ����д������ݲ������Թ���ڸ������ֽ�ޱ仯����ʪ����ֽ����ɫ��Ϊ��ɫ�����²���ֽ�ȱ�� ���ɴ�˵��������̼��������������̼����ˮ��Ӧ���ܶȱȿ����� �� |
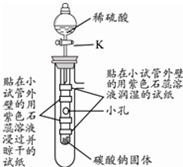
��3�� |
 |
��Һ©���IJ������ͻ���K����ˮע�������ι��У� |
�۲쵽�������� ����ʯ��ˮ�ͱ����������Һ�о��й������� �����ִ������ԭ����������������ˮ���ȣ���ʯ�ҵ��ܽ�����¶����߶����� ���������ˮ���ȣ�����ص��ܽ�����¶ȵĽ��Ͷ����� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
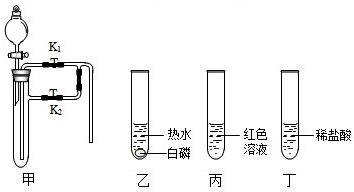
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
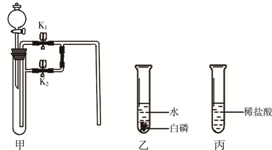 ������ij��ȤС��ͬѧ����2��Сʵ�飮����ʵ�����ݻش��������⣨�����Ĺ̶�װ���Ѿ�ʡ�ԣ���
������ij��ȤС��ͬѧ����2��Сʵ�飮����ʵ�����ݻش��������⣨�����Ĺ̶�װ���Ѿ�ʡ�ԣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱���з�̨���п�һģ��ѧ�Ծ��������棩 ���ͣ�̽����
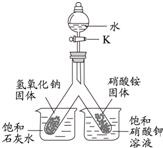
������ij��ȤС��ͬѧ����3��Сʵ�飮����ʵ�����ݻش��������⣨�����Ĺ̶�װ���Ѿ�ʡ�ԣ���
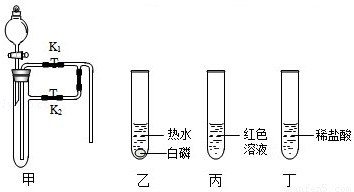
��1��������Ϊ�������̣����Ӽ���װ�ã��� ������ء�K1���͡�K2���IJ��������ӷ�Һ©������м�������˫��ˮ����ʵ�����֤����ȼ��ȼ����Ҫ�������Ӵ������з�����Ӧ�Ļ�ѧ����ʽΪ�� ����
��2��������Ϊˮ������Ϊ��ɫ��Һ�����Ӽױ�����K1���ر�K2���ӷ�Һ©������м���ijҩƷ����ֻ������Һ�ɺ�ɫ��Ϊ��ɫ��д�����з�����Ӧ�Ļ�ѧ����ʽ�� ����
��3��������Ϊ������̼��K1��K2�رգ������Ӽ����ӷ�Һ©������м��������ij���ʯ��ˮ��һ��ʱ���K1������ʵ������м��й۲쵽�������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����̨��һģ ���ͣ��ʴ���
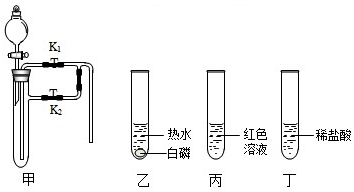
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com