ijĶ¬Ń§ÓūÅäÖĘÖŹĮæ·ÖŹżĪŖ7%µÄŃĪĖįČÜŅŗ£¬ĻÖÓŠŅ»ĘæŃĪĖįČÜŅŗ£¬ĪŖ²ā¶ØĘæÄŚČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż£¬Č”øĆČÜŅŗ73gÓŚÉÕ±ÖŠ£¬¼ÓČė12.5gŹÆ»ŅŹÆ£¬Ē”ŗĆĶźČ«·“Ó¦£¬³ĘµĆÉÕ±ÄŚŹ£ÓąĪļÖŹµÄ×ÜÖŹĮæŹĒ81.1g£®Ēėøł¾ŻŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©·¢Éś»Æѧ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£Ø2£©Éś³ÉĘųĢåµÄÖŹĮæŹĒ
£Ø3£©øł¾ŻĘųĢåµÄÖŹĮæĮŠ³öĒó½āĀČ»ÆĒāÖŹĮæµÄ±ČĄżŹ½ĪŖ
£Ø4£©ĘæÄŚŃĪĖįČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżŹĒ
£Ø5£©ČōČ”Ņ»¶ØĮæµÄĘæÄŚČÜŅŗÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ7%µÄŃĪĖįČÜŅŗ50g£¬Šč¼ÓČėĖ®µÄÖŹĮæŹĒ g£®
”¾“š°ø”æ
·ÖĪö£ŗÕāŹĒŅ»µĄ×ŪŗĻĢā£¬æ¼²éĮĖѧɜ¶ŌÖŹĮæŹŲŗć¶ØĀɵĥķ½ā”¢»Æѧ·½³ĢŹ½µÄ¼ĒŅäŗĶŹéŠ“”¢»Æѧ·½³ĢŹ½µÄ¼ĘĖćŅŌ¼°ČÜÖŹÖŹĮæ·ÖŹżµÄ¼ĘĖć£¬»¹ŃµĮ·ĮĖѧɜČÜŅŗĻ”ŹĶµÄ¼ĘĖćÄÜĮ¦£®
½ā“š£ŗ½ā£ŗ£Ø1£©Ļ”ŃĪĖįŗĶĢ¼ĖįøĘ·“Ӧɜ³ÉĀČ»ÆøĘ”¢Ė®”¢¶žŃõ»ÆĢ¼£¬»Æѧ·½³ĢŹ½ĪŖ£ŗCaCO
3+2HCl=CaCl
2+H
2O+CO
2”ü£¬ĖłŅŌ“š°øĪŖ£ŗCaCO
3+2HCl=CaCl
2+H
2O+CO
2ӟ
£Ø2£©Éś³ÉĘųĢåµÄÖŹĮæµČÓŚ·“Ó¦Ē°ĪļÖŹµÄÖŹĮæ×ÜŗĶ¼õČ„·“Ó¦ŗóÉÕ±ÄŚĪļÖŹµÄÖŹĮæ×ÜŗĶ£ŗ73g+12.5g-81.1g=4.4g£¬¹Ź“š°øĪŖ£ŗ4.4g
£Ø3£©ÉčĀČ»ÆĒāµÄÖŹĮæĪŖx£¬
CaCO
3+2HCl=CaCl
2+H
2O+CO
2ӟ
73 44
x 4.4g
ĮŠ±ČĄżŹ½
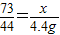
¹Ź“š°øĪŖ
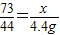
£Ø4£©ÓÉ
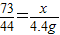
½āµĆx=7.3g£¬ŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ£ŗ
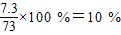
£¬¹Ź“š°øĪŖ£ŗ10%
£Ø5£©ŅŖĒó¼ÓĖ®µÄÖŹĮæŠėĻČĒóŠčŅŖŌČÜŅŗµÄÖŹĮ棬ÉčŌČÜŅŗÖŹĮæĪŖx Ōņ
50g×7%=x?10%
Ēó³ö x=35g
Ōņ¼ÓĖ®µÄÖŹĮæĪŖ50g-35g=15g£¬
¹Ź“š°øĪŖ£ŗ15g
µćĘĄ£ŗµŚ£Ø1£©Ģā-µŚ£Ø4£©ĢāŹĒ»ł“”ĢāѧɜÄܹ»Ķعż¼ņµ„µÄĖ¼æ¼±Č½ĻæģµÄ“š³öĄ“£¬µŚ£Ø5£©ĢāŹĒŅ»µĄ¾ßÓŠŅ»¶ØĖ¼Ī¬Éī¶ČµÄĢāÄ棬ŅŖĻČĖ¼æ¼¼ÓĖ®ŹĒŌŚŌČÜŅŗµÄ»ł“”ÉĻ¼ÓĖ®µ½50æĖ£¬ÕāŃł¾ĶŠčŅŖ¼ĘĖć³öŌĻČČÜŅŗµÄÖŹĮæ£¬Č»ŗó¾ĶÓČŠ¶ų½āĮĖ£®
æĘÄæ£ŗ³õÖŠ»Æѧ
Ą“Ō“£ŗ2011ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÖŠæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£ØĮł£©£Ø½āĪö°ę£©
ĢāŠĶ£ŗĢīæÕĢā
ijĶ¬Ń§ÓūÅäÖĘÖŹĮæ·ÖŹżĪŖ7%µÄŃĪĖįČÜŅŗ£¬ĻÖÓŠŅ»ĘæŃĪĖįČÜŅŗ£¬ĪŖ²ā¶ØĘæÄŚČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż£¬Č”øĆČÜŅŗ73gÓŚÉÕ±ÖŠ£¬¼ÓČė12.5gŹÆ»ŅŹÆ£¬Ē”ŗĆĶźČ«·“Ó¦£¬³ĘµĆÉÕ±ÄŚŹ£ÓąĪļÖŹµÄ×ÜÖŹĮæŹĒ81.1g£®Ēėøł¾ŻŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©·¢Éś»Æѧ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£Ø2£©Éś³ÉĘųĢåµÄÖŹĮæŹĒ
£Ø3£©øł¾ŻĘųĢåµÄÖŹĮæĮŠ³öĒó½āĀČ»ÆĒāÖŹĮæµÄ±ČĄżŹ½ĪŖ
£Ø4£©ĘæÄŚŃĪĖįČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżŹĒ
£Ø5£©ČōČ”Ņ»¶ØĮæµÄĘæÄŚČÜŅŗÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ7%µÄŃĪĖįČÜŅŗ50g£¬Šč¼ÓČėĖ®µÄÖŹĮæŹĒg£®
²éæ““š°øŗĶ½āĪö>>

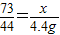
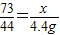
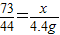 ½āµĆx=7.3g£¬ŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ£ŗ
½āµĆx=7.3g£¬ŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ£ŗ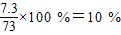 £¬¹Ź“š°øĪŖ£ŗ10%
£¬¹Ź“š°øĪŖ£ŗ10%

