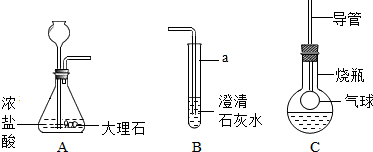
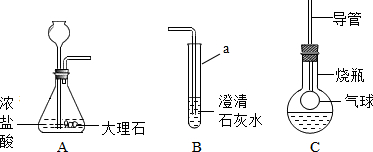
��ͼ��ij����С���ͬѧ��Ƶ�ʵ������ȡCO
2�����������ʵ�װ��ʾ��ͼ
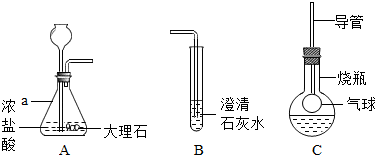
��ش���������
��1������a��ָ������������
��ƿ
��ƿ
��Aװ���з����Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
������A�г���©�����ɷ�Һ©�����ŵ���
���Ʒ�Ӧ�ķ�����ֹͣ
���Ʒ�Ӧ�ķ�����ֹͣ
��
��2����A�в���������ͨ�뵽B��һ�����δ�����б���ǣ�����ͬѧ��Ϊ����Ũ����ӷ�����HCl����������£�
���û�ѧ����ʽ��ʾΪ��Ca��OH��
2+CO
2=CaCO
3��+H
2O CaCO
3+2HCl=CaCl
2+H
2O+CO
2��
��Ľ��ͻ��ɸ���ݣ�����һ����ѧ����ʽ��ʾ��
2HCl+Ca��OH��2=CaCl2+2H2O
2HCl+Ca��OH��2=CaCl2+2H2O
��
��3���Ż�ͬѧ����ƿ�ռ�A�в���������������м��������ɫ����M��Һ���Cװ�ã���������ƿ�е������������Һʼ����ɫ������M������
NaOH����KOH��
NaOH����KOH��
����дһ�����ʵĻ�ѧʽ����
��4�������һ����������֤Cװ���з�Ӧ���������к���CO
32-��
| ���� |
���� |
���� |
ȡ��Ӧ�����Һ�����������еμ�
BaCl2��Һ��������ϡ���� BaCl2��Һ��������ϡ���� |
�а�ɫ�������ɻ�����ð�� �а�ɫ�������ɻ�����ð�� |
Cװ���з�Ӧ���������к���CO32- |
��5����A��ҩƷ���ɹ���������Һ�Ͷ������̣���Ӧ�Ļ�ѧ����ʽΪ
��




 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�