��2010?�������ʼ죩ijУ������ѧ��ȤС���ڽ�ʦָ���¡����Բⶨ����ڶ�����̼������������������£�
[������Ϣ]ij��������������=��ij����������/�����������������100%
[������]����������ڣ����ɣ���ʵ���ҳ��û�ѧ������ҩƷ
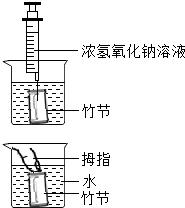
[ʵ�鲽��]��ͼ��
����ȡһ��ڣ���ֱ��û��ˮ�У�������϶���һС�ף���ע������С�������������ע��1-2mLŨ����������Һ��
������Ĵָ���������ף���ס�϶�С�ף�ȡ����ڣ�������
�����ٽ���ڽ�û��ˮ�У��ɿ�Ĵָ�����ӣ�
����ȡ����ڣ�������һ����һС�ף��ռ������Һ�壬��������ΪL
3��
������������½�û��ˮ�У���ˮ������ں�ȡ������������ˮ�����ΪL
4��
������ȡ����ظ���������¼���ݣ����±���
| �� �� |
�� |
�� |
�� |
�� |
�� |
| �����CO2�����/mL |
2.6 |
5.9 |
4.2 |
5.6 |
6.2 |
| ��������������/mL |
82 |
137 |
135 |
195 |
195 |
| �����CO2���������/% |
3.71 |
4.31 |
|
2.87 |
3.10 |
��1�����袡�У�������Ӧ�Ļ�ѧ��Ӧ����ʽΪ
2NaOH+CO2=Na2CO3+H2O
2NaOH+CO2=Na2CO3+H2O
��
��2�����袤�У���������һ����һС�ס���Ŀ����
�ڴ���ѹ�������£�����ȡ������ڵ�Һ��
�ڴ���ѹ�������£�����ȡ������ڵ�Һ��
��
��3�����袤�����У����L
3��L
4���õ��Ļ�ѧ������
��Ͳ
��Ͳ
��L
3��L
4�Ĺ�ϵ��L
3��
��
L
4���������=����������
��4���ϱ��ո��е�������
3.11
3.11
��
[ʵ�����]��5�������CO
2��ƽ���������
����
����
������ڡ���С�ڡ���������CO
2�����������
[ʵ����չ]��6��С����Ϊ�����ֻ�Ƚ������CO
2�������CO
2��������Ĵ�С�����м�ʵ����ܴﵽĿ�ģ�С�ϵ�ʵ�鷽��������
��ע�����ֱ��ȡ���������ںͿ��������壬Ȼ���ټ����ֱ��ȡ�����������������Һ������ȡע������ʾ�����ܹ��Ƚ϶��߶�����̼�����������С
��ע�����ֱ��ȡ���������ںͿ��������壬Ȼ���ټ����ֱ��ȡ�����������������Һ������ȡע������ʾ�����ܹ��Ƚ϶��߶�����̼�����������С
��д��ʵ������������ۣ���
��7����ȤС���ڽ�ʦָ���»����Բⶨ�������O
2�����������10%-12.5% ��Χ�ڣ��ݴˣ���ȤС���Ʋ⡰�����CO
2��O
2�����������ͬ�ڿ�������ԭ�������ֲ��Ĺ�������йأ�



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

 [������Ϣ]ij��������������=��ij����������/�����������������100%
[������Ϣ]ij��������������=��ij����������/�����������������100%