
���� �����������Ƶ����ʷ����ش�����������һ�ּ���������̼��Ӧ���������Ƶ��ܽ�����¶ȵ����߶���С��
��� �⣺��1����������������һ�ּ���������̼��Ӧ������̼��Ƴ��������Լ�ͬѧ��һ֧�Թ��ڼ�������ʯ��ˮ����ͨ�������̼���壬�Թ��ڳ��ֻ��ǣ���Ӧ�Ļ�ѧ����ʽ�ǣ�Ca��OH��2+CO2�TCaCO3��+H2O��
��2�������������Ƶ��ܽ�����¶ȵ����߶���С����ͬѧ��һ֧�Թ��ڼ�������ʯ��ˮ���ٽ��Թ��Լ��ȣ��۲쵽���������ͬѧ��ʵ��������ͬ��ԭ�����¶����ߣ������������ƣ�
��3��������ʯ������ˮ��Ӧ�������������ƣ�������������ˮ�����ԣ���ͬѧ��һ֧�Թ��ڼ�������ʯ��ˮ�������Թ��м���������ʯ�ң���ȴ��ԭ�¶�ʯ��ˮ����ǣ�����ԭ�¶ȵı�����Һ����Һ����ɲ��䣮���ԣ�AD��ȷ��
�ʴ�Ϊ����1��Ca��OH��2+CO2�TCaCO3��+H2O����2���¶����ߣ������������ƣ���3��AD��
���� ������ѶȲ����˽��������Ƶ����ʡ��ܽ�����¶ȱ仯������ǽ����Ĺؼ���


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ���ȷ��� | B�� | ����ƿ���� | C�� | ��ֲ����� | D�� | ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
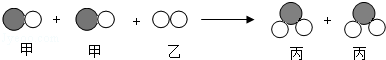 �ס��������ʷ�����Ӧ����ʾ��ͼ��ͼ����˵����ȷ���ǣ�������
�ס��������ʷ�����Ӧ����ʾ��ͼ��ͼ����˵����ȷ���ǣ�������| A�� | �÷�Ӧǰ��ԭ�Ӹ������ֲ��� | |
| B�� | �÷�Ӧ���ڸ��ֽⷴӦ | |
| C�� | ���ҵ���Է�������֮�͵��ڱ�����Է������� | |
| D�� | �ͱ���ͬ��Ԫ�صĻ��ϼ۾���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����к�������Ԫ���ǵ�Ԫ�� | |
| B�� | �ؿ��������������Ľ���Ԫ������Ԫ�� | |
| C�� | ���к���Ԫ�� | |
| D�� | ���к��⡢��Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
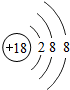
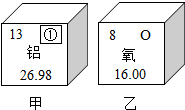 ��1������ԭ�ӽṹ�����֪ʶ��ͼʾ��Ϣ��գ�
��1������ԭ�ӽṹ�����֪ʶ��ͼʾ��Ϣ��գ� ��
��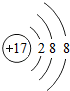 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.2% | B�� | 2.5% | C�� | 10.4% | D�� | 11.1% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
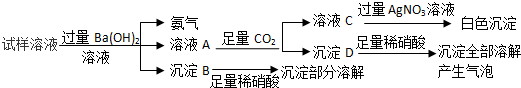
| A�� | ������Һ�п��ܺ���NaNO3��BaCl2 | |
| B�� | ����D�Ļ�ѧʽΪBaCO3 | |
| C�� | ������Һ��һ������NH4NO3��MgCl2��Na2SO4 | |
| D�� | ��ʵ�����漰�Ļ�ѧ��Ӧ��һ���Ǹ��ֽⷴӦ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com