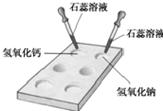
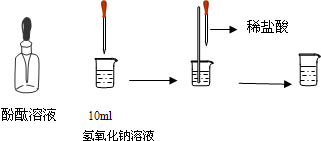
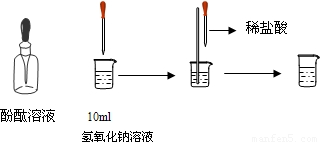
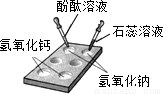
Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½²¢ĢīæÕ£®
£Ø1£©ŹµŃéŹŅÓĆ¹żŃõ»ÆĒāČÜŅŗÖĘČ”ŃõĘų£ŗ
£ŗ³£¼ÓČėµÄŅ»ÖÖŗŚÉ«¹ĢĢåµÄ×÷ÓĆŹĒ
“ß»Æ×÷ÓĆ
“ß»Æ×÷ÓĆ
£®
£Ø2£©ĀĮŗĶĻ”ŃĪĖį·“Ó¦£ŗ
2Al+6HCl=2AlCl3+3H2ӟ
2Al+6HCl=2AlCl3+3H2ӟ
£»øĆ·“Ó¦ŹōÓŚ
·ÅČČ
·ÅČČ
£ØĢī”°·ÅČČ”±»ņ”°ĪüČČ”±£©·“Ó¦£®
£Ø3£©Į¶Ģś³§ŅŌ³ąĢśæóĪŖŌĮĻĮ¶Ģś£ŗ
£»øĆ·“Ó¦ĄąŠĶ
²»ŹĒ
²»ŹĒ
£ØĢī”°ŹĒ”±»ņ”°²»ŹĒ”±£©ÖĆ»»·“Ó¦£®
£Ø4£©ĶĀĢŹÜČČ·Ö½ā£ŗ
Cu
2£ØOH£©
2CO
3H
2O+2CuO+CO
2ӟ
Cu
2£ØOH£©
2CO
3H
2O+2CuO+CO
2ӟ
£»øĆ·“Ó¦ĄąŠĶµÄŹĒ
·Ö½ā·“Ó¦
·Ö½ā·“Ó¦
£®
£Ø5£©¼ų±šµŖĘųŗĶ¶žŃõ»ÆĢ¼³£ÓƵÄŅ»ÖÖČÜŅŗµÄĆū³ĘŹĒ
³ĪĒåµÄŹÆ»ŅĖ®
³ĪĒåµÄŹÆ»ŅĖ®
£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
CO2+Ca£ØOH£©2ØTCaCO3”ż+H2O
CO2+Ca£ØOH£©2ØTCaCO3”ż+H2O
£®