
����ʵ��װ��ͼ�ش����⣮
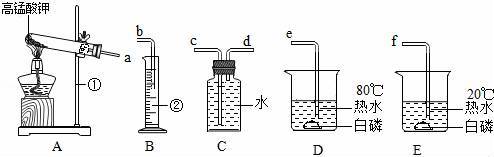

��1��д������������ƣ���������
��2��д��Aװ���Թ��з�����Ӧ�Ļ�ѧ����ʽ������
��3����Ҫ��ȡ�������ⶨ������轫����A��B��Cװ�ý�����װ���ܿڵ�����˳��Ϊa��������������b������Cװ���ռ�����������Ϊ����������
��4���������������ֱ�ͨ��D��Eװ���У��۲쵽D�еİ���ȼ�գ�E�а��ײ�ȼ�գ���һ��ʵ˵����ȼ��ȼ�ձر���������������
�����㡿��������ȡװ�ã�����װ�ã��������ռ���������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ��ȼ����ȼ�յ�������
��ר�⡿ʵ���Լ���⣮
����������1������ʵ���ҳ�����������ʶ������⣻
��2������������ȷֽ���������غͶ������̺��������ݴ���д����ʽ��
��3����Ҫ��ȡ������ͨ����ˮ�����ⶨ���ռ������������������ʵ��װ�ýӿڵ���ȷ����˳��Ϊa��d��e��b��
��4������ʵ��������ͼ���ṩ����Ϣ����ȼ����Ҫ��������
����𡿽⣺��1������ʵ���ҳ�����������ʶ������⣬��������̨��
��2������������ȷֽ���������غͶ������̺���������ѧ��Ӧ����ʽ��2KMnO4
 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
��3����Ҫ��ȡ������ͨ����ˮ�����ⶨ���ռ������������������ʵ��װ�ýӿڵ���ȷ����˳��Ϊa��d��c��b����Ϊ������������ˮ����˿�����Cװ���ռ��������ʴ�Ϊ��d��c����������ˮ��
��4��D�еİ���ȼ�գ�E�еİ��ײ�ȼ�գ����¶��������������һ����ֻ��˵�����¶ȴﲻ����ԭ��˵����ȼ��ȼ�ձر����������¶ȴﵽ��ȼ����Ż�㣻
�ʴ�Ϊ��
��1������̨��
��2��2KMnO4
 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
��3��d��c����������ˮ��
��4���¶ȴﵽ��ȼ����Ż�㣻
�����������ճ������������ƣ����������ȡ������ԭ����Ҫ����ȼ�յ����������п�ȼ��¶ȴﵽ�����ʵ��Ż�㡢�������Ӵ���֪�����Ʊ�����ʵ�����Ʒ�����ֻ������һ������
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ˮ��Դ����ʶ�У�������ǣ�������
A��ˮͨ����̬�仯ʵ����Ȼѭ��
B��������������ˮ���Եõ��㹻�ĵ�ˮ�����ԡ���Լ��ˮ���������Ѿ���ʱ��
C����ѧ��������ʹ�û��ʺ�ũҩ����ˮԴ�ص�ˮ������ϸ�
D����Ȼ��ˮ�������������ˡ�������ɱ�������Ⱦ������̣��ɱ��������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ԭ����ʵ�������������һ��Ԫ������һ��Ԫ�ؼ���ʵ�������������Ӳˮ��������������ܶ�䣬Ҳ�����ǵ���������ܴ�Σ���������г�������������Ӳˮ�����ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ����������᳣���¶�����Ӧ����ϡ�����ڼ���ʱ���Է�����Ӧ�����ݷ���ʽ3Cu+8HNO3
 3Cu��NO3��2+4H2O+2X�����Ʋ�X�Ļ�ѧʽΪ��������
3Cu��NO3��2+4H2O+2X�����Ʋ�X�Ļ�ѧʽΪ��������
A��NO2 B��NO C��N2O3 D��N2O5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ƻ�ѧ������գ�
��1�����������������������������
��2��һ��笠�����������
��3�����������������Ԫ�صĻ��ϼ���2����
��4���Ȼ�����Һ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�����ƿ��ʢ������X����ͷ�ι���ʢ��Һ��Y������ѹ��ͷ�ι�ʹҺ�������ƿ�У���һ��ʱ�� ��ɼ�С����a�������±��еĸ������ʲ�����������������
��ɼ�С����a�������±��еĸ������ʲ�����������������
| X | Y | |
| A | CO | Ca��OH��2��Һ |
| B | CO2 | NaOH��Һ |
| C | HCl | Ca��OH��2��Һ |
| D | SO2 | NaOH��Һ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʼ䲻�ܷ������ֽⷴӦ����
A.̼��ƺ�ϡ���� B.��������Һ���Ȼ�����Һ
C.�Ȼ�����Һ���������Һ D.�Ȼ�����Һ������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���������������ȷ���ǣ�������
A������������ȼ��ʱ���������ĵ���ɫ����
B����˿��������ȼ��ʱ���������䣬���ɺ�ɫ����
C��þ���ڿ�����ȼ��ʱ������ҫ�۵İ⣬���ɰ�ɫ����
D�������ڿ�����ȼ��ʱ������������ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ƻ���и���ƻ���ᣬƻ����Ļ�ѧʽΪC4H4O4������˵����ȷ���ǣ�������
A��ƻ�����к���12��ԭ��
B��ƻ�������Է�������Ϊ112
C��ƻ������̼Ԫ�ص���������ԼΪ41.4%
D��ƻ������C��H��O��Ԫ�ص�������Ϊ4��4��4
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com