Θ®2013?―τ–≈œΊΡΘΡβΘ©ΫώΧλ «–ΓΟςΑ÷Α÷ΒΡ…ζ»’Θ°
Θ®1Θ©¬η¬ηΈΣΑ÷Α÷’εΝΥ¬ζ¬ζ“Μ±≠ΑΉΨΤΘ§–ΓΟς‘Ε‘ΕΨΆΈ≈ΒΫΝΥΨΤΒΡΧΊ βœψΈΕΘ§«κ”ΟΈΔΝΘΒΡΙέΒψΫβ ΆΈ≈ΒΫœψΈΕΒΡ‘≠“ρ
ΨΤΨΪΖ÷Ή”‘Ύ≤ΜΕœ‘ΥΕ·
ΨΤΨΪΖ÷Ή”‘Ύ≤ΜΕœ‘ΥΕ·
Θ°
Θ®2Θ©Α÷Α÷Βψ»ΦΝΥ…ζ»’ά·÷ρΘ§–μ‘ΗΚσ¥σΦ““ΜΤπΫΪά·÷ρ¥ΒΟπΘ°’β÷÷œ®Οπά·÷ρΜπ―φΒΡ‘≠άμ «
ΫΒΒΆΈ¬Ε»÷ΝΩ…»ΦΈοΉ≈ΜπΒψ“‘œ¬
ΫΒΒΆΈ¬Ε»÷ΝΩ…»ΦΈοΉ≈ΜπΒψ“‘œ¬
Θ°
Θ®3Θ©–ΓΟς”Ο―ΙΥξ«°ΗχΑ÷Α÷¬ρΝΥ“ΜΦΰ―ρΟΪ…άΘ°»’≥Θ…ζΜν÷–Ω…“‘”Ο
ΉΤ…’Θ®Μρ»Φ…’ΜρΒψ»ΦΘ©
ΉΤ…’Θ®Μρ»Φ…’ΜρΒψ»ΦΘ©
ΒΡΖΫΖ®Φλ―ιΘ°
Θ®4Θ©¬η¬ηΥΒΘ§ΒΑΗβ…œ≤ψΒΡΡΧ”Ά «”Οœ ≈ΘΡΧΦ”ΙΛΒΟΒΫΒΡΘ§≈ΘΡΧ÷–ΗΜΚ§ΒΡ”Σ―χΥΊΈΣ
ΒΑΑΉ÷
ΒΑΑΉ÷
Θ°
Θ®5Θ©ΑϋΉΑΒΑΗβΒΡΥήΝœ¥ϋ τ”Ύ
”–ΜζΚœ≥…
”–ΜζΚœ≥…
≤ΡΝœΘ§”ΟΚσ»γΥφ“βΕΣΤζΜα‘λ≥…
ΑΉ…ΪΈέ»ΨΘ®ΜρΜΖΨ≥Έέ»ΨΜρΈέ»ΨΘ©
ΑΉ…ΪΈέ»ΨΘ®ΜρΜΖΨ≥Έέ»ΨΜρΈέ»ΨΘ©
Θ°



 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
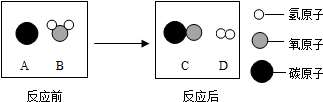 Θ®2013?±ΘΩΒœΊΡΘΡβΘ©Ρή‘¥ΓΔΜΖΨ≥”κ»ΥάύΒΡ…ζΜνΚΆ…γΜαΖΔ’ΙΟή«–œύΙΊΘ°
Θ®2013?±ΘΩΒœΊΡΘΡβΘ©Ρή‘¥ΓΔΜΖΨ≥”κ»ΥάύΒΡ…ζΜνΚΆ…γΜαΖΔ’ΙΟή«–œύΙΊΘ°