
ijĒāŃõ»ÆøĘÖŠŗ¬Ģ¼ĖįøĘŌÓÖŹ”£³ĘȔъĻøµÄøĆѳʷ12.4g·ÅČė׶ŠĪĘæÖŠ£¬¼ÓČė32.6gµÄĖ®£¬³ä·ÖÕńµ“ŠĪ³ÉŠü×ĒŅŗ£¬ŌŁĻņ׶ŠĪĘæÖŠÖšµĪµĪ¼ÓŃĪĖįŹ¹Ęä³ä·Ö·“Ó¦ÖĮĪŽĘųÅŻ²śÉś”£²āµĆ¼ÓČėŃĪĖįµÄÖŹĮæÓė׶ŠĪĘæÖŠµÄĪļÖŹµÄÖŹĮæ¹ŲĻµČēĻĀ±ķŹ¾”£
| ¼ÓČėŃĪĖįµÄÖŹĮæg | 0 | 25 | 37.5 |
| ׶ŠĪĘæÖŠĪļÖŹµÄÖŹĮæ | 45 | 70 | 80.3 |
£Ø1£©¼ÓČėŃĪĖįµÄÖŹĮæŌŚ0—25gŹ±£¬ ÓėŃĪĖį·¢Éś·“Ó¦”£
£Ø2£©·“Ó¦²śÉś¶žŃõ»ÆĢ¼ÖŹĮæĪŖ g”£
£Ø3£©Ēóѳʷ֊ĒāŃõ»ÆøʵÄÖŹĮæ·ÖŹż£ØŠ“³ö¼ĘĖć¹ż³Ģ£¬¾«Č·µ½0.1%£©”£
”¾“š°ø”æ£Ø1£©ĒāŃõ»ÆøĘ£Ø2£©2.2g
£Ø3£©½ā£ŗÉčøĆѳʷ֊Ģ¼ĖįøʵÄÖŹĮæĪŖx
CaCO3+2HCl=CaCl2+H2O+CO2ӟ
100 44
X 2.2g
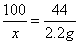
X=5g
øĆѳʷ֊ĒāŃõ»ÆøʵÄÖŹĮæ·ÖŹżĪŖ£ŗ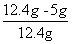 ”Į100%”Ö59.7%
”Į100%”Ö59.7%
“š£ŗѳʷ֊ĒāŃõ»ÆøʵÄÖŹĮæ·ÖŹżĪŖ59.7%”£
”¾½āĪö”æÓɱķÖŠŹż¾Ż·ÖĪöæÉÖŖµ±¼ÓČėŃĪĖįµÄÖŹĮæĪŖ0——25Ź±£¬ŹĒĒāŃõ»ÆøĘÓėŃĪĖį·¢ÉśÖŠŗĶ·“Ó¦£¬µ±¼ÓČė37.5gŃĪĖįŹ±²śÉśµÄ¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ£ŗ37.5g+12.4g+32.6g-80.3g=2.2g£¬øł¾Ż»Æѧ·½³ĢŹ½æÉŅŌ¼ĘĖć³öѳʷ֊Ģ¼ĖįøʵÄÖŹĮ棬øł¾ŻŃłĘ·µÄÖŹĮæĒó³öĒāŃõ»ÆøʵÄÖŹĮæ·ÖŹż”£


 ±øÕ½ÖŠæ¼ŗ®¼ŁĻµĮŠ“š°ø
±øÕ½ÖŠæ¼ŗ®¼ŁĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
×é³ÉČĖĢå×ŌÉķµÄŌŖĖŲÓŠ50¶ąÖÖ,ĘäÖŠŃõ”¢Ģ¼”¢Ēā”¢µŖ¼øÖÖŌŖĖŲŅŌĖ®”¢ĢĒĄą”¢ÓĶÖ¬”¢µ°°×ÖŹŗĶĪ¬ÉśĖŲµÄŠĪŹ½“ęŌŚ,ĘäÓąŌŖĖŲŅŌĪŽ»śŃĪµÄŠĪŹ½“ęŌŚ”£
(1)Ķ¬ÖŹĮæµÄÓĶÖ¬”¢µ°°×ÖŹŌŚĢåÄŚĶźČ«Ńõ»ÆŹ±,·Å³öÄÜĮæ¶ąµÄŹĒ””””””””;
(2)Ź³Īļµķ·ŪŌŚČĖĢåÄŚÓėĖ®·¢ÉśŅ»ĻµĮŠ·“Ó¦,×īÖÕ±ä³ÉĘĻĢŃĢĒ,ĘĻĢŃĢĒµÄ»ÆѧŹ½ŹĒ””””””””””;
(3)¶ąŹżĪ¬ÉśĖŲŌŚČĖĢåÄŚ²»ÄÜŗĻ³É,ŠčŅŖ“ÓŹ³ĪļÖŠÉćČ”,Čōȱ·¦””””””””””»įŅżĘš»µŃŖ²””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČÕ³£Ź¹ÓĆµÄ½šŹō²ÄĮĻ£¬“󶹏żŹōÓŚŗĻ½š”£»ĘĶŹĒŅŌŠæ×÷Ö÷ŅŖĢķ¼ÓŌŖĖŲµÄĶŗĻ½š”£
¢Å³ąĶ(Cu2O)ŹĒĪŅ¹ś¹Å“śÖĘČ”»ĘĶµÄŅ»ÖÖŌĮĻ”£Cu2OÖŠĶŌŖĖŲÓėŃõŌŖĖŲµÄÖŹĮæ±ČŹĒ  ”£
ӣ
¢ĘµÆæĒµÄ»ĘĶÖ»ŗ¬ÓŠŠæŗĶĶ”£½«22 gµÆæĒ·ÅŌŚŹ¢ÓŠ100 gĻ”ĮņĖįµÄÉÕ±ÖŠ(ĮņĖį×ćĮæ)£¬µ±µÆæĒ²»ŌŁČܽāŗó£¬ÉÕ±ÖŠ»ģŗĻĪļµÄÖŹĮæŹĒ121. 8 g”£¼ĘĖć:
¢Ł²śÉśĒāĘųµÄÖŹĮ攣
¢ŚµÆæĒÖŠĶµÄÖŹĮ攣
¢Ū·“Ó¦ŗóĖłµĆČÜŅŗÖŠZnSO4µÄÖŹĮæ·ÖŹż(¼ĘĖć½į¹ū±£ĮōŅ»Ī»Š”Źż)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖĮĖ²ā¶Øij“æ¼īѳʷ(ŗ¬ÉŁĮæĀČ»ÆÄĘŌÓÖŹ)ÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Č”øĆѳʷÓė×ćĮæĻ”ŃĪĖįŌŚÉÕ±ÖŠ·“Ó¦£¬ÓŠ¹ŲŹµŃ鏿¾ŻČēĻĀ±ķ
| ·“Ó¦Ē° | ·“Ó¦ŗó | ||
| ŹµŃ鏿¾Ż | ÉÕ±ŗĶĻ”ŃĪĖįµÄÖŹĮæ£Æg | “æ¼īѳʷµÄÖŹĮæ£Æg | ÉÕ±ŗĶĘäÖŠ»ģŗĻĪļµÄÖŹĮæ£Æg |
| 120 | 12 | 127£®6 |
Ēė¼ĘĖć£ŗ
(1)·“Ӧɜ³É¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ g”£
(2)øĆ“æ¼īѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ¶ąÉŁ”£(ĒėŠ“³ö¼ĘĖć¹ż³Ģ)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«Ņ»¶ØÖŹĮæµÄ¼×ĶéŗĶŅ»Ńõ»ÆĢ¼µÄ»ģŗĻĘųĢåŌŚ×ćĮæµÄŃõĘųÖŠ³ä·ÖČ¼ÉÕ£¬½«Éś³ÉĪļŅĄ“ĪĶØČėŹ¢ÓŠ×ćĮæÅØĮņĖįŗĶĒāŃõ»ÆÄĘČÜŅŗµÄĻ“ĘųĘ棬ŹµŃé²āµĆ×°ÓŠÅØĮņĖįµÄĻ“ĘųĘæŌöÖŲ5.4æĖ£¬×°ÓŠĒāŃõ»ÆÄĘČÜŅŗµÄĻ“ĘųĘæŌöÖŲ8.8æĖ”£ŹŌ¼ĘĖć£ŗ
£Ø1£©»ģŗĻĘųĢåÖŠ¼×ĶéµÄÖŹĮæ£ØŠ“³öĶźÕūµÄ¼ĘĖć¹ż³Ģ£©
£Ø2£©»ģŗĻĘųĢåÖŠ¼×ĶéŗĶŅ»Ńõ»ÆĢ¼µÄÖŹĮæÖ®±ČĪŖ______£¬»ģŗĻĘųĢåÖŠĢ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲµÄÖŹĮæÖ®±ČĪŖ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
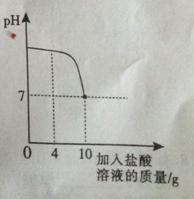
ŹµŃéŹŅÖŠÓŠŅ»Ęæ±źĒ©±»øÆŹ“µÄŃĪĖį,Š”ĄöĪŖĮĖ²ā¶ØČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż,ŌŚÉÕ±ÖŠÅäÖĘĮĖ8æĖ10%µÄĒāŃõ»ÆÄĘČÜŅŗ,Č»ŗóĶłÉÕ±ÖŠµĪ¼ÓøĆŃĪĖį£¬·“Ó¦¹ż³ĢÖŠČÜŅŗµÄpHÓėµĪČėŃĪĖįµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÅäÖĘ8æĖ10%µÄĒāŃõ»ÆÄĘČÜŅŗ£¬ŠčŅŖĖ®µÄÖŹĮæĪŖ g£»
£Ø2£©¼ĘĖćøĆŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹż£»£ØŠ“³ö¼ĘĖć¹ż³Ģ£©
£Ø3£©µ±µĪČė4æĖŃĪĖįŹ±£¬ÉÕ±ÄŚČÜŅŗÖŠÄĘŌŖĖŲµÄÖŹĮæĪŖ g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŠĀŅ©ÅĮĄĆ×Ī¤×¢ÉäŅŗŹĒÖĪĮĘH7N9ĒŻĮ÷øŠµÄÓŠŠ§Ņ©ĪļÖ®Ņ»£¬ÅĮĄĆ×Ī¤µÄ»ÆѧŹ½ĪŖ£ŗC15H28N4O4£®ŹŌ¼ĘĖć£ŗ
£Ø1£©C15H28N4O4µÄŅ»øö·Ö×ÓÖŠ¹²ÓŠ””””øöŌ×Ó£®
£Ø2£©C15H28N4O4ÖŠĒāŌŖĖŲÓėŃõŌŖĖŲµÄÖŹĮæ±ČŹĒ”” ””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖĮĖ²ā¶ØŅ»·ŻĢśĆ¾ŗĻ½š»ģŗĻ·ŪÄ©ÖŠĢśµÄÖŹĮæ·ÖŹż£¬Ä³Š£»ÆѧŠĖȤŠ”×éČ”øĆѳʷ8g·ÖĖÄ“Ī¼ÓČėŹ¢ÓŠ100gĪ“ÖŖÅØ¶ČµÄĮņĖįČÜŅŗµÄÉÕ±£ØŅŃÖŖÉÕ±ÖŹĮæĪŖ50g£©ÖŠ£¬³ä·Ö·“Ó¦ŗ󣬲āµĆÉÕ±ŗĶÉÕ±ÄŚĪļÖŹµÄÖŹĮæŹż¾Ż¼ĒĀ¼ČēĻĀ£ŗ
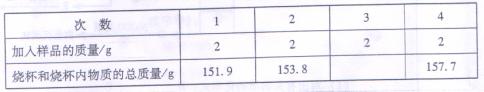
£Ø1£©±ķÖŠµŚČż“Ī¼ÓČė»ģŗĻ·ŪÄ©ŗó£¬ÉÕ±ŗĶÉÕ±ÄŚČÜŅŗµÄ×ÜÖŹĮæŹĒ g£»
£Ø2£©ĖłÓĆĮņĖįČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹżŹĒ £»
£Ø3£©»ģŗĻ·ŪÄ©ÖŠĢśµÄÖŹĮæ·ÖŹżŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µ±Ē°ĪŅŹŠ²æ·ÖÖŠŠ”ѧĶĘŠŠ”°Ńō¹āŹ³ĢĆ”±¹¤³Ģ”£ĻĀ±ķĪŖijŠ£Ź³ĢĆijĢģĪē²Ķ²æ·ÖŹ³Ę×”£
| Ö÷Ź³ | »ē²Ė | Źß²Ė |
| Ć×·¹ | ŗģÉÕÅ£Čā | ³“ŗśĀܲ·”¢³“»Ę¹Ļ |
(1)Ź³Ę×ÖŠø»ŗ¬µ°°×ÖŹµÄŹĒ________£¬ø»ŗ¬Ī¬ÉśĖŲµÄŹĒ________(ĢīÉĻ±ķÖŠµÄŅ»ÖÖÖ÷Ź³»ņ²ĖĆū)£¬Ć×·¹ÖŠÖ÷ŅŖŗ¬ÓŠČĖĢå±ŲŠčµÄÓŖŃųĖŲŹĒ______________”£
(2)Ź³ĢĆÖŠĆ¹±äµÄ“óĆ×________”£
A.æÉŅŌŹ³ÓĆ””B.ÕōÖóŗóæÉŅŌŹ³ÓĆ””C.¾ų¶Ō²»ÄÜŹ³ÓĆ
(3)Ķ¬Ń§øųѧŠ£Ź³ĢƵÄĻĀĮŠ½ØŅé²»ŗĻĄķµÄŹĒ________”£
A.ŌŚÖ÷Ź³ÖŠ²¹³ä“ÖĮø”””” B.¶ąĢį¹©ÓĶÕØŹ³Īļ
C.ŹŹµ±Ģį¹©Ė®¹ū
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com