
 ������Լռ���������
������Լռ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����о��꼶��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�̽����
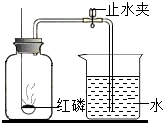
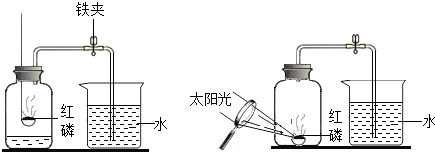
23��ijͬѧ����ͼ��ʾ��װ�����ⶨ���������������ĺ�����ʵ�顣

��1�������ڼ���ƿ��ȼ�գ��ɹ۲쵽��������
��2������Ϩ����ȴ���ɼк۲쵽��������
��3��ȼ�պ���ƿ�ڵ���Ҫ������Ҫ�ǵ��������ϱ�ʵ��
�Ʋ�������ʣ�
�������ʣ�
��ѧ���ʣ�
��4��ʵ���з��ֽ��뼯��ƿ�е�ˮδ�ﵽԤ���ĸ߶ȣ������ԭ��
��5��ʵ�������С��ͬѧ��ʵ������˷�˼������˸Ľ��ķ���������ͼ��ʾ����
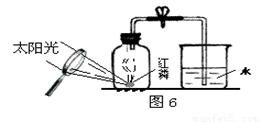
����Ϊ�����Ľ����ŵ��У�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ʡ�����ۺϸ�ϰ��ѧ�Ծ��������棩 ���ͣ�̽����
�����е�SO2�����Ϳ���������ĺ���������g/cm3��ʾ��������Ҫ�Ŀ�������ָ�ꡣ�ڹ�ҵ�����Ϲ涨�������ж����������������ŷ�Ũ�Ȳ��ó���0.02 mg/L����2004�꽭��ʡ����״��������ָ��������ʡ2004�깤ҵ(��Ҫ���ȵ糧)���������ŷ�����Ϊ1.24��106t��ȫʡ������Ⱦ�Ƚ����ء���������ɷַ��֣���ˮ���������������������Ϊ����Լռ������������61.9����
��һ��(1)��������ˮ�� ��(��:�ᡢ�����)��ԭ���� ��
(2)���ڴ�����SO2��NOx(NO��NO2)�ĺ����������ߣ���ת��ΪH2SO4�ͺ�HNO3����ˮ������γɵġ���ʡ��Ҫ�����������ꡣ��ʡ���������γɹ���������;����һ����SO2�ڿ����е�Ʈ���������������±���������ΪSO3������ˮ�������ᣬд���йصķ�Ӧ��ѧ����ʽ �� ��
��һ����SO2����ˮ���������ᣬ�ٱ������е��������������ᣬд���йصķ�Ӧ��ѧ����ʽ �� ������SO2�������dz��������ʺ�һ��;��������Ҫ�γɹ��̡�
��3����ȤС��ͬѧȡ�ս����ȵ糧��������ˮ���вⶨ��ÿ������Ӳ�һ��pH�����������±���ʾ��
|
�ⶨʱ�� |
5��05 |
5��10 |
5��15 |
5��20 |
5��25 |
5��30 |
5��35 |
|
pH |
4.95 |
4.94 |
4.94 |
4.88 |
4.86 |
4.85 |
4.85 |
�����������ݱ仯������Եó��Ľ����� ����Դ˽��ۣ���²����е�ԭ��Ϊ ����ϴ���ʯ�����긯ʴ����ʵ������Ϊ̼�ᡢ���ᡢ������������ǿ������˳��Ϊ__________________________��
������SO2 ������ʹƷ����Һ�����������Һ��ɫ��

�ش��������⣺
��1���������װ��C�������ԵIJ����ǣ��رջ���b��Ȼ��________________________ ��
������________________��˵��װ��C���������á�
��2��ʵ������Na2SO3�����������Һ��ȡSO2���壬Ӧѡ��__________��ѡ��A����C���������巢��װ�ã���ѡ����һװ�õ������� ��
��3��С���ͬѧ��A��Cװ���е���һ���ÿ�״FeS�����ϡ������ȡH2S���壬��Ӧ�ķ���ʽΪ_________________ ��
��4��SO2����ͨ��Dװ��ʱ������____________________ ��ͨ��Eװ��ʱ������________________ ��SO2��H2S��Bװ���з�Ӧ������һ���������һ�ֵ��ʣ���Ӧ�ķ���ʽ�� ��
��5��F������������___________��F��ʢ��NaOH���壬������
��������Ϊ�ⶨij�ط��Ŀ�����SO2�Ϳ���������ĺ������������ϵ�֪�����ú���һ���������Һ�ⶨ�����е�SO2��������Ӧ����ʽ�ǣ�SO2��I2��2H2O��H2SO4��2HI����ͬѧ���������ͼ��ʾ��ʵ��װ�ã�
��Ӧ������װ�òⶨ�����е�SO2�����Ϳ���������ĺ��������ⶨ�������٣���λ��cm3/min���⣬����Ҫ�ⶨ����ǰ�����������������ʢ���������������� ��
����֪���ⵥ������ˮ��KI�����������ˮ�е��ܽ�ȡ�
����Э����ͬѧ���ϡ����Һ�����ƣ�
��һ����ȷ��ȡ1.27g�ⵥ�ʼ����ձ��У� ��
�ڶ�����������ˮʹ֮��ȫ�ܽ⣬Ȼ���ˮ����Һ���Ϊ1000 mL��
���������ӵڶ���������Һ�У�ȡ��10.00mL��Һ����ϡ����100 mL��
����ͬѧ�������м���װ�òⶨ�����е�SO2������ȷȡ50mL���������õĵ���Һ��ע����ͼ��ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼����������n����
������ͬѧ�IJ�����ȷ�ģ���ͬѧ�����Ĵ���Ϊ80�Σ�����˵���õؿ����е�SO2�����Ƿ�����ŷű�����д��������̣�����3�֣�

�Ǹ�С��ͬѧ��Ϊ��Щ����Ҫ���ų��ķ������д�����Ϊ�˴ﵽ���Ч������ʵ���ҽ�����ʵ��ģ�⣬����Ϊ����Ϊ�����ղ�����SO2 ���壬��ʹ��ҩƷӦ����________�������Ļ�ѧ��Ӧ����ʽ______________________________________
��������˼�������������װ�ã���Ϊ�������ֲ��ܲ���______,��Ϊ����װ�ö�����________���á�

A B C D E F G H I J
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
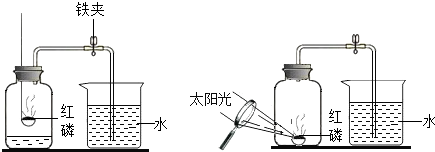
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����й�����ѧ���꼶���ϣ���һ���¿���ѧ�Ծ��������棩 ���ͣ������
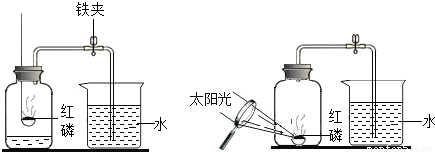
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com