

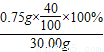 ×100%=1.0%Ј¬
×100%=1.0%Ј¬


| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
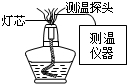 ЈЁ2004?БъСТЈ©ѕЖѕ«µЖµЖСж·ЦСжРДЎўДЪСжєННвСжИэёцІї·ЦЈ®ОЄМЅѕїµЖСжОВ¶ИЈ¬їЖСРИЛФ±УГМШКвµДІвОВЧ°ЦГЅшРРКµСйЈЁИзНј5Ј©Ј¬Ѕб№ыИзПВЈЁМЅН·О»ЦГКЗЦёІвОВМЅН·АлµЖРѕµДґ№Ц±ёЯ¶ИЈ©Ј®
ЈЁ2004?БъСТЈ©ѕЖѕ«µЖµЖСж·ЦСжРДЎўДЪСжєННвСжИэёцІї·ЦЈ®ОЄМЅѕїµЖСжОВ¶ИЈ¬їЖСРИЛФ±УГМШКвµДІвОВЧ°ЦГЅшРРКµСйЈЁИзНј5Ј©Ј¬Ѕб№ыИзПВЈЁМЅН·О»ЦГКЗЦёІвОВМЅН·АлµЖРѕµДґ№Ц±ёЯ¶ИЈ©Ј®| »р Сж | Сж РД | ДЪ Сж | Нв Сж | ||||
| МЅН·О»ЦГЈЁcmЈ© | 0.5 | 1.5 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 |
| »рСжОВ¶ИЈЁЎжЈ© | 537 | 670 | 775 | 806 | 801 | 750 | 667 |
| ЖЅѕщОВ¶ИЈЁЎжЈ© | 603 | 794 | 708 | ||||
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com