½ā£ŗ
£Ø1£©·½°øŅ»£ŗæÉŃ”ŌńNaClČܽāŌŚÕōĮóĖ®ÖŠ½ųŠŠÅäÖĘ£¬ŠčŅŖNaClµÄÖŹĮæ=100g”Į0.9%=0.9g£¬ŠčŅŖĖ®µÄÖŹĮæ=100g-0.9g=99.1g£»
·½°ø¶ž£ŗæÉŃ”ŌńĻņ0.5%µÄNaClČÜŅŗ¼ÓČėNaCl£¬¼ĢŠųČܽā¶ųÅäÖĘ£¬ÉčŠčŅŖĀČ»ÆÄʵÄÖŹĮæĪŖx£¬x+£Ø100g-x£©”Į0.5%=100g”Į0.9%£¬½āµĆx=0.4g£¬ŠčŅŖ0.5%µÄNaClČÜŅŗ=100g-0.4g=99.6g£»
·½°øČż£ŗŃ”Ōń1.5%µÄNaClČÜŅŗŗĶ0.5%µÄNaClČÜŅŗ½ųŠŠ»ģŗĻÅäÖĘ£¬ÉčŠčŅŖ1.5%µÄNaClČÜŅŗÖŹĮæĪŖy£¬y”Į1.5%+£Ø100g-y£©”Į0.5%=100g”Į0.9%£¬½āµĆy=40g£¬ŠčŅŖ0.5%µÄNaClČÜŅŗ=100g-40g=60g£»
·½°øĖÄ£ŗŃ”Ōń1.5%µÄNaClČÜŅŗ¼ÓÕōĮóĖ®Ļ”ŹĶ½ųŠŠÅäÖĘ£¬ÉčŠčŅŖ1.5%µÄNaClČÜŅŗÖŹĮæĪŖz£¬z”Į1.5%=100g”Į0.9%£¬½āµĆz=60g£¬ŠčŅŖĖ®µÄÖŹĮæ=100g-60g=40g£»
£Ø2£©ÉčĀČ»ÆÄʵÄÖŹĮæĪŖx
NaCl+AgNO
3ØTAgCl”ż+NaNO
358.5 143.5
x 2.87g
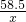
=
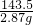
x=1.17g
øĆÉśĄķŃĪĖ®µÄČÜÖŹÖŹĮæ·ÖŹż=
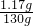
”Į100%=0.9%£¬·ūŗĻŅ½ÓƱź×¼£»
¹Ź“š°øĪŖ£ŗ
£Ø1£©·½°øŅ»£ŗŃ”ŌńNaClŗĶÕōĮóĖ®ÅäÖĘ£®ŠčŅŖNaClµÄÖŹĮæ=100g”Į0.9%=0.9g£¬ŠčŅŖĖ®µÄÖŹĮæ=100g-0.9g=99.1g£»
·½°ø¶ž£ŗŃ”ŌńNaClŗĶ²æ·Ö0.5%µÄNaClČÜŅŗÅäÖĘ£®ÉčŠčŅŖĀČ»ÆÄʵÄÖŹĮæĪŖx£¬x+£Ø100g-x£©”Į0.5%=100g”Į0.9%£¬½āµĆx=0.4g£¬ŠčŅŖ0.5%µÄNaClČÜŅŗ=100g-0.4g=99.6g£»
·½°øČż£ŗŃ”Ōń1.5%µÄNaClČÜŅŗŗĶ0.5%µÄNaClČÜŅŗ½ųŠŠÅäÖĘÉčŠčŅŖ1.5%µÄNaClČÜŅŗÖŹĮæĪŖy£¬y”Į1.5%+£Ø100g-y£©”Į0.5%=100g”Į0.9%£¬½āµĆy=40g£¬ŠčŅŖ0.5%µÄNaClČÜŅŗ=100g-40g=60g£»
·½°øĖÄ£ŗŃ”Ōń1.5%µÄNaClČÜŅŗŗĶÕōĮóĖ®½ųŠŠÅäÖĘ£¬ÉčŠčŅŖ1.5%µÄNaClČÜŅŗÖŹĮæĪŖz£¬z”Į1.5%=100g”Į0.9%£¬½āµĆz=60g£¬ŠčŅŖĖ®µÄÖŹĮæ=100g-60g=40g£»
£Ø2£©·ūŗĻŅ½ÓƱź×¼£®
·ÖĪö£ŗ£Ø1£©øł¾ŻÓūÅäÖĘ100gÉśĄķŃĪĖ®£¬ŠčŅŖ0.9gNaClŗĶ99.1gĖ®£¬ĄūÓĆĖłĢį¹©µÄŅ©Ę·£¬æɲÉČ”¹ĢĢåČܽā”¢ÅØČÜŅŗ¼ÓĖ®Ļ”ŹĶ”¢Ļ”ČÜŅŗ¼ÓČėĀČ»ÆÄĘ¼ĢŠųČܽā»ņÅØĻ”ČÜŅŗ½ųŠŠ»ģŗĻµČ·½·Ø£¬Č”ŹŹµ±Ņ©Ę·£¬Ź¹Ö®Āś×ćÅäÖĘČÜŅŗĖłŠčŅŖĖ®µÄĀČ»ÆÄʵÄÖŹĮ棬»ģŗĻ¾łŌČ¼“æÉ£»
£Ø2£©øł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬ÓÉÉś³ÉĀČ»ÆŅų³ĮµķµÄÖŹĮ棬¼ĘĖćøĆÉśĄķŃĪĖ®130gÖŠĖłŗ¬ĀČ»ÆÄʵÄÖŹĮ棬Ēó³öøĆÉśĄķŃĪĖ®ÖŠĀČ»ÆÄʵÄÖŹĮæ·ÖŹż£¬²¢Óė0.9%½ųŠŠ¶Ō±Č£¬ÅŠ¶ĻŹĒ·ń·ūŗĻŅŖĒó£®
µćĘĄ£ŗÅäÖĘČÜŅŗŅ»°ćæɲÉČ”¹ĢĢåĪļÖŹÖ±½ÓČܽā”¢ÅØČÜŅŗ¼ÓĖ®Ļ”ŹĶµÄ·½·Ø£¬Ņ²æÉŅŌ²ÉČ”Ļ”ČÜŅŗ¼ĢŠųČܽāøĆČÜÖŹ»ņÅØĻ”ČÜŅŗ»ģŗĻµÄ·½·ØÅäÖʶų³É£®

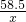 =
=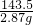 x=1.17g
x=1.17g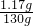 ”Į100%=0.9%£¬·ūŗĻŅ½ÓƱź×¼£»
”Į100%=0.9%£¬·ūŗĻŅ½ÓƱź×¼£»

 ³¬ÄÜѧµäÓ¦ÓĆĢāĢāæØĻµĮŠ“š°ø
³¬ÄÜѧµäÓ¦ÓĆĢāĢāæØĻµĮŠ“š°ø
 £Ø2013?¾£ÖŻÄ£Äā£©Ēė»Ų“šĻĀĮŠÓŠ¹ŲČÜŅŗµÄĪŹĢā£ŗ
£Ø2013?¾£ÖŻÄ£Äā£©Ēė»Ų“šĻĀĮŠÓŠ¹ŲČÜŅŗµÄĪŹĢā£ŗ