
����Ŀ����Դ�������������������ᷢչ������ء�
��1��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ�________.
��2��Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ�����壬�˹��̿���Ϊ��̼��ˮ�����ڸ��������µķ�Ӧ������ʾ��ͼ������ʾ��
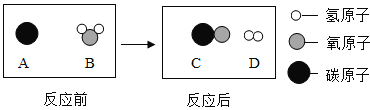
�÷�Ӧ�Ļ�ѧ����ʽΪ_________��������Ӧ����Ϊ________��
��3��Ϊ�������������ŷţ����ǻ���Ѱ�Ҳ���̼Ԫ�ص�ȼ�ϡ����о�����![]() ȼ�յIJ���û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ����
ȼ�յIJ���û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ����![]() �е�Ԫ�غ���Ԫ�ص�������Ϊ________��
�е�Ԫ�غ���Ԫ�ص�������Ϊ________��![]() ȼ�����ɿ����к������������ˮ����д���˷�Ӧ�Ļ�ѧ����ʽ__________��
ȼ�����ɿ����к������������ˮ����д���˷�Ӧ�Ļ�ѧ����ʽ__________��
���𰸡���Ȼ�� C+H2O![]() CO+H2 �û���Ӧ 14��3 4NH3+3O2
CO+H2 �û���Ӧ 14��3 4NH3+3O2![]() 2N2+6H2O
2N2+6H2O
��������
��1�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ���������Ȼ����
��2��������ģ��ͼ��֪��̼��ˮ�ڸ����·�Ӧ����һ����̼���������˷�Ӧ�ǵ��ʺͻ����ﷴӦ���ɵ��ʺͻ�����������û���Ӧ�����C+H2O![]() CO+H2 �û���Ӧ��
CO+H2 �û���Ӧ��
��3��![]() �е�Ԫ�غ���Ԫ�ص�������Ϊ14����1��3��=14��3��
�е�Ԫ�غ���Ԫ�ص�������Ϊ14����1��3��=14��3��![]() ȼ�����ɿ����к������������ˮ�������к������������ǵ��������Դ˷�Ӧ�Ļ�ѧ����ʽΪ��4NH3+3O2
ȼ�����ɿ����к������������ˮ�������к������������ǵ��������Դ˷�Ӧ�Ļ�ѧ����ʽΪ��4NH3+3O2![]() 2N2+6H2O�����14��3 4NH3+3O2
2N2+6H2O�����14��3 4NH3+3O2![]() 2N2+6H2O��
2N2+6H2O��


 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Dz�Ҷ֮�磬�����̲��еĵ������������Ѫѹ���ߡ����Ƚⶾ�������ȹ�Ч���仯ѧʽΪC76H52O46������˵����ȷ����
A.��������������Ԫ����ɵĻ����
B.1�������������76��̼ԭ�ӡ�52����ԭ�Ӻ�46����ԭ�ӹ���
C.���������Է�������Ϊ1700g
D.��������C��H��OԪ�ص�������Ϊ38��26��23
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ��Ӻ�ԭ����������������ȷ����
A.����ֻ���ɷ��ӹ���B.��ͬԭ�ӿ��ܹ��ɲ�ͬ�ķ���
C.��������һ������ԭ������D.��ѧ�仯�з�����Ŀһ�������仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ļ������ڹ�ũҵ�������й㷺��Ӧ�á�
(һ)����Ӧ�úͷ���
A���ҹ�������ʱ�ھ��������������Ϊͭ��֮˵���û�ѧ����ʽ��ʾ��ԭ����_____��
B�����������Ҫ����Ϊ��
Fe![]() Fe(OH)2
Fe(OH)2![]() Fe(OH)3
Fe(OH)3![]() Fe2O3xH2O
Fe2O3xH2O
(1)д��ת���ٵĻ�ѧ����ʽ_____��
(2)ת�������� Fe2O3xH2O���� x��_____(x Ϊ����)��
C.�������ֹ�������һ����ʩ_____��
(��)���Ļ�����Ӧ��
������(��Ҫ�ɷ���FeS2)����һ����Ҫ�Ļ���ԭ�ϣ��������Ʊ����������(ͼ1)��
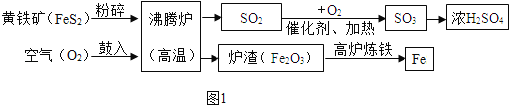
A����ҵ�Ͻ�����������Ŀ����_____��
B������������β���к��� SO2����ֱ���ŷſ��ܻ���ɻ���������_____��
C����¯�����Ļ�ѧ����ʽΪ_____��
D.150 �ֺ� FeS280%�Ļ����������������Ƶ� 98%��Ũ����_____�֡�
(��)����ұ����̽��
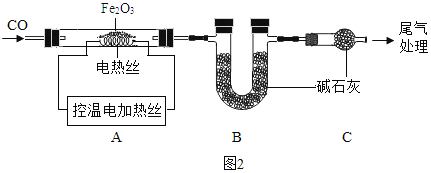
ȡ 24.0g Fe2O3 ��ĩ��С��ͬѧ��ͼ2װ��ģ������������÷�Ӧ�����ɷ֡�
���ϣ���ʯ�ҿ����� H2O �� CO2��
A������װ�ã���_____����װ��ҩƷ��
B��ʵ��ʱ��ͨ��CO��Ŀ����_____��
C������A���¶��� 700��������ȫ����ڣ�����ͨCO����ȴ��
(1)ͨ���ⶨװ�� B �й���������仯����ȷ���װ�� A ��ʣ�����������װ��C��������_____��
(2)��ֱ�Ӳ��װ�� A ��ʣ���������Ϊ19.2g����װ�� B �й���Ӧ����_____g��
D��������A��ʣ����� 19.2g ΪFe�� FexOy �Ļ��������м���������ϡ H2SO4��� ��Ӧ���� H20.3g��
(1)������ Fe ������Ϊ_____g��
(2)FexOy �Ļ�ѧʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס����������ʵ��ܽ��������ͼ��ʾ����˵����ȷ���ǣ� ��

A.�����ʵ��ܽ�ȴ��������ʵ��ܽ��
B.���ҵı�����Һ��![]() �����µ�
�����µ�![]() ��ʱ���о�������
��ʱ���о�������
C.![]() ��ʱ�����ҵı�����Һ��10g�������ʵ��������
��ʱ�����ҵı�����Һ��10g�������ʵ��������
D.![]() ��ʱ�����Ҹ�30g�ֱ����100gˮ�г���ܽ⣬���γɱ�����Һ
��ʱ�����Ҹ�30g�ֱ����100gˮ�г���ܽ⣬���γɱ�����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����20��ʱ����20g����ع������50gˮ�У��������ܽ������4.2g����ع���δ�ܽ⡣����д���пհף�
��1��������Һ��20��ʱ����ص�________��Һ������͡������͡���
��2��20��ʱ����ص��ܽ��Ϊ__________��
��3��������Һ������ص�������������Ϊ________����ȷ��0.1����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���û�ѧ���ű�ʾ��
��1���ؿ��к������Ľ���Ԫ��_____ ��
��2��2����ԭ��_____ ��
��3��m��þ����_____ ��
��4��һ����ԭ��_____ ��
��5���ܱ��ֶ�����̼��ѧ���ʵ�����____ ��
��6������_____��
��7��2�����������________��
��8������������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ij� ��Ԫ�أ�ÿ�ձ��������㹻���ĸơ�Ŀǰ�г��ϵIJ���ҩ���ܶ࣬��ͼ��ij��Ʒ�� �IJ���ҩƷ�IJ���˵���顣 ��ش��������⣺

��1��CaCO3����Է���������___________��
��2��CaCO3�и�Ԫ�ص���������Ϊ___________��
��3����ÿƬ��Ƭ������Ϊ0.5g�����Ƭ�и�Ԫ�ص���������Ϊ___________��
��4�����ÿ��3�Σ�ÿ��2Ƭ��ÿ�������Ԫ�ص�����Ϊ___________g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������Ʒ����ڿ��������ױ���ת��Ϊ̼�������ֳ�ȡһ�����ڿ����е��ռ���Ʒ10.6g��ȫ��������ˮ�����100g��Һ�������еμ���������Ϊ8.55%������������Һ��������������������������Һ��������ϵ��ͼ��ʾ��
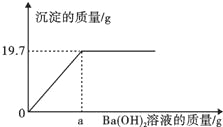
��ͨ������ش�[
��1�����ռ���Ʒ���ʵij̶�Ϊ ������ֱ��ʡ���ȫ�����ʡ�����
��2��a= g��
��3���������պôﵽ�������ʱ����Һ��������������Ϊ���٣���д��������̣���������ȷ��0.01%��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com