
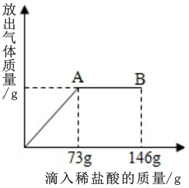
 ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠÓÉNa2CO3ŗĶNaCl ×é³ÉµÄ¹ĢĢå»ģŗĻĪļ25g£¬ĻņĘäÖŠÖš½„µĪ¼ÓČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖį£¬·Å³öĘųĢåµÄÖŹĮæÓėµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£ØŅŃÖŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗNa2CO3+2HCl=2NaCl+CO2”ü+H2O£©£®Ēė·ÖĪöĒśĻßĶ¼ŗó»Ų“šĻĀĮŠĪŹĢā£ŗ
ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠÓÉNa2CO3ŗĶNaCl ×é³ÉµÄ¹ĢĢå»ģŗĻĪļ25g£¬ĻņĘäÖŠÖš½„µĪ¼ÓČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖį£¬·Å³öĘųĢåµÄÖŹĮæÓėµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£ØŅŃÖŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗNa2CO3+2HCl=2NaCl+CO2”ü+H2O£©£®Ēė·ÖĪöĒśĻßĶ¼ŗó»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö ÓÉÓŚøų³öĮĖĻūŗĵÄŃĪĖįµÄÖŹĮæ¼°ĘäÖŹĮæ·ÖŹż£¬æÉŅŌøł¾ŻŃĪĖįÖŠHClµÄÖŹĮæŗĶ¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ĒóĖć¶ŌÓ¦µÄĢ¼ĖįÄʵÄÖŹĮæŗĶÉś³ÉµÄĀČ»ÆÄʵÄÖŹĮ棬×īŗóĒóĖćĘäÖŹĮæ·ÖŹż£®¶ŌÓŚŃĪĖįµĪ¼Ó¹ż³ĢµĆµ½µÄĶ¼Ļó£¬ŗÜČŻŅ×擳öµ±µĪ¼ÓŃĪĖįµ½73gŹ±ĪŖĒ”ŗĆĶźČ«·“Ó¦£¬¼Óµ½146gŹ±ĪŖŃĪĖį¹żĮ棬“ĖŹ±pHŠ”ÓŚ7£®
½ā“š ½ā£ŗøł¾ŻĶ¼æÉŅŌ擳ö£¬µ±µĪ¼ÓŃĪĖįÖĮ73gŹ±Ņ²¾ĶŹĒAµćŹ±£¬“ĖŹ±ĘųĢå“ļµ½×ī“óĮ棬Ņ²¾ĶŹĒĒ”ŗĆĶźČ«·“Ó¦£¬“ĖŹ±ČÜŅŗpHµČÓŚ7£¬ČÜÖŹÖ»ÓŠĀČ»ÆÄĘ£®¶ų¼ĢŠųµĪ¼ÓŃĪĖįŹ±£¬ÓÉÓŚŃĪĖįŹ£Óą£¬ĖłŅŌ“ĖŹ±pHŠ”ÓŚ7£¬ČÜÖŹĪŖHClŗĶNaCl£®
ŌŚAµćŹ±ĻūŗĵÄHClµÄÖŹĮæĪŖ73g”Į10%=7.3g£®
ÉčĻūŗÄ7.3gHClŹ±·“Ó¦µÄĢ¼ĖįÄʵÄÖŹĮæĪŖx£¬Éś³ÉµÄ¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖy
Na2CO3+2HClØT2NaCl+H2O+CO2”ü
106 73 44
x 7.3g y
$\frac{106}{x}$=$\frac{73}{7.3g}$=$\frac{44}{y}$
xØT10.6g
y=4.4g
µ½“ļBĘųĢ嶞Ńõ»ÆĢ¼µÄÖŹĮæŅ²ŅĄ¾ÉŹĒ4.4g£®
ĖłŅŌ»ģŗĻĪļÖŠĀČ»ÆÄʵÄÖŹĮæĪŖ25g-10.6gØT14.4g
»ģŗĻĪļÖŠ ĀČ»ÆÄʵÄÖŹĮæ·ÖŹżĪŖ$\frac{14.4g}{25g}$”Į100%ØT57.6%
¹Ź“š°øĪŖ£ŗ£Ø1£©=£» NaCl£»£¼£»
£Ø2£©7.3£» 4.4£» 4.4£»
£Ø3£©57.6%£®
µćĘĄ øł¾Ż»Æѧ·½³ĢŹ½¼ĘĖ揱£¬µŚŅ»ŅŖÕżČ·ŹéŠ“»Æѧ·½³ĢŹ½£¬µŚ¶žŅŖŹ¹ÓĆÕżČ·µÄŹż¾Ż£¬µŚČż¼ĘĖć¹ż³ĢŅŖĶźÕū£®


 ŠĀæĪ±źæģĄÖĢįÓÅŹī¼Ł×÷ŅµÉĀĪ÷ĀĆÓĪ³ö°ęÉēĻµĮŠ“š°ø
ŠĀæĪ±źæģĄÖĢįÓÅŹī¼Ł×÷ŅµÉĀĪ÷ĀĆÓĪ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ¹żĀĖ | B£® |  ¼ÓČČŅŗĢå | ||
| C£® |  ³żČ„COÖŠµÄĖ®ÕōĘū | D£® |  ĮæČ”9.3mLŅŗĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | A | B | C | D |
| ×÷Īļ | ²čŹ÷ | ĆŽ»Ø | øŹ ²Ż | Ė®µ¾ |
| ŹŹŅĖ×÷ĪļÉś³¤µÄpH | 5.0”«5.5 | 6.0”«6.8 | 7.2”«8.5 | 6.0”«7.0 |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ŗĻ½šĆÅ“° | B£® |  ĖÜĮĻĘæ | C£® |  ÄįĮś±³°ü | D£® |  Ęū³µĀÖĢ„ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
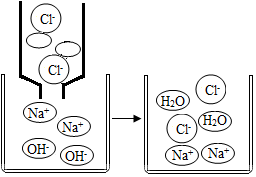 Ķ¼ĪŖĒāŃõ»ÆÄĘČÜŅŗÓėĻ”ŃĪĖįĒ”ŗĆĶźČ«·“Ó¦µÄĪ¢¹ŪŹ¾ŅāĶ¼£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
Ķ¼ĪŖĒāŃõ»ÆÄĘČÜŅŗÓėĻ”ŃĪĖįĒ”ŗĆĶźČ«·“Ó¦µÄĪ¢¹ŪŹ¾ŅāĶ¼£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | +1 | B£® | +2 | C£® | +3 | D£® | +4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¶žŃõ»ÆĢ¼ | B£® | Ļ”ÓŠĘųĢå | C£® | ŃõĘų | D£® | µŖĘų |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com