
 ��ϸ�Ķ�����ij�ּӸ�ʳ�ΰ�װ���ϵIJ������֣����ش����⣮
��ϸ�Ķ�����ij�ּӸ�ʳ�ΰ�װ���ϵIJ������֣����ش����⣮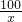 =
=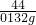
 ��100%=1.2%
��100%=1.2%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���ϱ����Ȼ��ơ�ʳ��̼��ơ������ ��������500g �ɷֱ����Ȼ��ơ�88% �ƣ���Ca�ƣ���0.5��1.3��% �⣨��I�ƣ���20��50��mg/kg��1���������ʳ�����Ƿ�̼��ƣ��ڼ�ͥ�����п�ѡ�� ��2��Ϊ�˲ⶨ������̼��Ƶĺ�����ȡ10g��������������ˮ���������������ᣬ����0.132g������̼����������ʧ������˼Ӹ�ʳ����̼��Ƶ���������Ϊ���٣� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����  ��ϸ�Ķ�����ij�ּӸ�ʳ�ΰ�װ���ϵIJ������֣����ش����⣮ ��ϸ�Ķ�����ij�ּӸ�ʳ�ΰ�װ���ϵIJ������֣����ش����⣮��1���������ʳ�����Ƿ�̼��ƣ��ڼ�ͥ�����п�ѡ�� ��2��10g����Ʒ�к�̼��Ƶ������� ��3������Ʒ�к���Ԫ�ص����������������ݼ������жϴ˼Ӹ�ʳ�κ���Ԫ�ص����������Ƿ�ϸ���ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2���� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ����� ����ϸ�Ķ�����ij�ּӸ�ʳ�ΰ�װ���ϵIJ������֣�����ջش����⣮
|