
(11Зж) аЁРіКЭМИЮЛЭЌбЇдкЪЕбщЪвгУЯТСазАжУжЦШЁМИжжЦјЬхЁЃ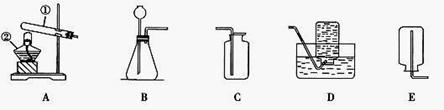
ЂХаДГіЭМжагаБъКХЕФвЧЦїЕФУћГЦЃКЂй ЃЛ Ђк ЁЃ
ЂЦаЁРігУИпУЬЫсМижЦШЁбѕЦјЃЌЫћбЁдёЩЯЪізАжУжаЕФAКЭ ЃЈЬюзжФИЃЉНјаазщзАЁЃаДГіИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ ЁЃ,гУДЫЗНЗЈжЦбѕЦјЪБЃЌЪдЙмПкЗХвЛЭХУоЛЈЕФзїгУЪЧ_________________________________ЁЃ
ЂЧЪЕбщЪвбЁгУBКЭCзАжУПЩжЦШЁЕФЦјЬхЪЧ ЃЌаДГіЪЕбщЪвжЦШЁИУЦјЬхЕФЛЏбЇЗНГЬЪН ЁЃ ЃЈ4ЃЉЪЕбщЪвЭЈГЃгУШчгвЭМЫљЪОЕФЯДЦјзАжУЖдбѕЦјНјааИЩдяЃЌЯДЦјЦПРяУцЬюзАЕФвЉЦЗПЩвдЪЧ ЁЃ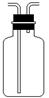
| AЃЎХЈСђЫс | BЃЎЧтбѕЛЏФЦШмвК | CЃЎЩњЪЏЛв | DЃЎТШЛЏФЦ |
 ПкЫуЬтПЈББОЉИОХЎЖљЭЏГіАцЩчЯЕСаД№АИ
ПкЫуЬтПЈББОЉИОХЎЖљЭЏГіАцЩчЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК2011-2012бЇФъЙуЖЋЪЁУЏУћЪажаПМФЃФтПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКМђД№Ьт
(11Зж) аЁРіКЭМИЮЛЭЌбЇдкЪЕбщЪвгУЯТСазАжУжЦШЁМИжжЦјЬхЁЃ

ЂХаДГіЭМжагаБъКХЕФвЧЦїЕФУћГЦЃКЂй ЃЛ Ђк ЁЃ
ЂЦаЁРігУИпУЬЫсМижЦШЁбѕЦјЃЌЫћбЁдёЩЯЪізАжУжаЕФAКЭ ЃЈЬюзжФИЃЉНјаазщзАЁЃаДГіИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ ЁЃ,гУДЫЗНЗЈжЦбѕЦјЪБЃЌЪдЙмПкЗХвЛЭХУоЛЈЕФзїгУЪЧ_________________________________ЁЃ
ЂЧЪЕбщЪвбЁгУBКЭCзАжУПЩжЦШЁЕФЦјЬхЪЧ ЃЌаДГіЪЕбщЪвжЦШЁИУЦјЬхЕФЛЏбЇЗНГЬЪН ЁЃ ЃЈ4ЃЉЪЕбщЪвЭЈГЃгУШчгвЭМЫљЪОЕФЯДЦјзАжУЖдбѕЦјНјааИЩдяЃЌЯДЦјЦПРяУцЬюзАЕФвЉЦЗПЩвдЪЧ ЁЃ

AЃЎХЈСђЫс BЃЎЧтбѕЛЏФЦШмвК CЃЎЩњЪЏЛв DЃЎТШЛЏФЦ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com