
£Ø6·Ö£©»Æѧъ¾æĪļÖŹµÄ×é³É”¢½į¹¹”¢ŠŌÖŹ”¢±ä»ÆÖ®¼äµÄ¹ŲĻµ”£
£Ø1£©æĘѧ¼ŅĶعżŃŠ¾æĖ®µÄ±ä»ÆČĻŹ¶ĮĖĖ®µÄ×é³É”£ĻĀĶ¼ĪŖµē½āĖ®µÄ¼ņŅ××°ÖĆ£¬ŹŌ¹Ü¢ŁÖŠ²śÉśµÄĘųĢåŹĒ £¬ÓÉ“ĖŹµŃéµĆ³öĖ®ŹĒÓÉ ×é³ÉµÄ£Ø2£©×é³ÉŗĶŠŌÖŹÓŠ×ÅĆÜĒŠµÄĮŖĻµ£¬ĖįČÜŅŗ¾ßÓŠĻąĖʵĻÆѧŠŌÖŹŹĒŅņĪŖĖįÖŠ¶¼ŗ¬ÓŠ ”£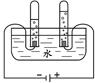
£Ø3£©½į¹¹¾ö¶ØŠŌÖŹ”£ŃŠ¾æ·¢ĻÖŗ¬ÓŠ”°¹żŃõ»ł”±£Ø”ŖO”ŖO”Ŗ£©µÄĪļÖŹ¾ßÓŠŗÜĒæµÄŃõ»ÆŠŌ£¬æÉŅŌ×öĪŖɱ¾śĻū¶¾¼Į”£¾Ż“ĖĶĘ²ā£¬ĻĀĮŠĪļÖŹÖŠ£¬æÉÓĆ×÷ɱ¾śĻū¶¾¼ĮµÄŹĒ (ĢīŹż×ÖŠņŗÅ)”£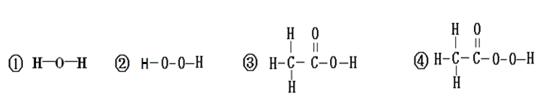
£Ø4£©Ķعż·ÖĪö×é³ÉŗĶ½į¹¹£¬ĪŅĆĒæÉŅŌŌ¤²āĪļÖŹµÄijŠ©ŠŌÖŹ”£¹ŲÓŚNaHSO4ŠŌÖŹµÄĻĀĮŠĶĘ²ā£¬ŗĻĄķµÄŹĒ £ØĢīŹż×ÖŠņŗÅ£©”£
¢ŁĘäĖ®ČÜŅŗÄÜÓė¼Ų·¢ÉśÖĆ»»·“Ó¦£¬µĆµ½½šŹōÄĘ
¢ŚĘäĖ®ČÜŅŗÄÜŹ¹×ĻÉ«ŹÆČļŹŌŅŗ±äŗģ
¢ŪĘäĖ®ČÜŅŗÄÜÓėŠæ·“Ӧɜ³ÉĒāĘų
¢ÜĘäĖ®ČÜŅŗÄÜÓėĻõĖį±µ·“Ӧɜ³ÉĮņĖį±µ³Įµķ”££ØĢįŹ¾£ŗĮņĖį±µ²»ČÜÓŚĖ®£¬Ņ²²»ČÜÓŚĖį£©
£Ø1£©ĒāĘų ĒāŌŖĖŲŗĶŃõŌŖĖŲ £Ø2£©ĒāŌŖĖŲ £Ø3£©¢Ś¢Ü £Ø4£©¢Ś¢Ū¢Ü
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©µē½āĖ®µÄ¼ņŅ××°ÖĆ£¬ŹŌ¹Ü¢ŁÖŠ²śÉśµÄĘųĢåŹĒĒāĘų£¬ÓÉ“ĖŹµŃéµĆ³öĖ®ŹĒÓÉĒāŌŖĖŲŗĶŃõŌŖĖŲ×é³ÉµÄ”££Ø2£©×é³ÉŗĶŠŌÖŹÓŠ×ÅĆÜĒŠµÄĮŖĻµ£¬ĖįČÜŅŗ¾ßÓŠĻąĖʵĻÆѧŠŌÖŹŹĒŅņĪŖĖįÖŠ¶¼ŗ¬ÓŠĒāŌŖĖŲ£»£Ø3£©½į¹¹¾ö¶ØŠŌÖŹ”£ŃŠ¾æ·¢ĻÖŗ¬ÓŠ”°¹żŃõ»ł”±£Ø”ŖO”ŖO”Ŗ£©µÄĪļÖŹ¾ßÓŠŗÜĒæµÄŃõ»ÆŠŌ£¬æÉŅŌ×öĪŖɱ¾śĻū¶¾¼Į£¬¹ŹæÉÓĆ×÷ɱ¾śĻū¶¾¼ĮµÄŹĒ»ÆѧŹ½ÖŠŗ¬ÓŠ¹żŃõ»łµÄĪļÖŹ¢Ś¢Ü£¬£Ø4£©Ķعż·ÖĪö×é³ÉŗĶ½į¹¹£¬ĪŅĆĒæÉŅŌŌ¤²āĪļÖŹµÄijŠ©ŠŌÖŹ”£¹ŲÓŚNaHSO4ŠŌÖŹ¢ŚĘäĖ®ČÜŅŗÄÜŹ¹×ĻÉ«ŹÆČļŹŌŅŗ±äŗģ¢ŪĘäĖ®ČÜŅŗÄÜÓėŠæ·“Ӧɜ³ÉĒāĘų¢ÜĘäĖ®ČÜŅŗÄÜÓėĻõĖį±µ·“Ӧɜ³ÉĮņĖį±µ³Įµķ”£
æ¼µć£ŗĪļÖŹµÄ×é³É”¢½į¹¹”¢ŠŌÖŹ


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ÆѧŹĒŅ»ĆÅŅŌŹµŃéĪŖ»ł“”µÄæĘѧ£¬½čÖśæĘѧµÄŹµŃé·½·Ø£¬ĪŅĆĒæÉŅŌ“Ó²»Ķ¬½Ē¶ČÕżČ·µŲČĻŹ¶ĪļÖŹ£®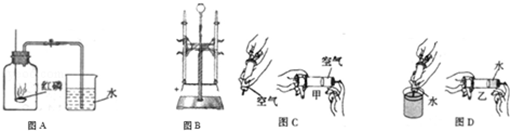
ŹµŃéŅ»£ŗĢ½¾æĪļÖŹµÄ×é³É
£Ø1£©Ķ¼AĖłŹ¾ŹµŃéæÉÓĆÓŚ²ā¶ØæÕĘųµÄ×é³É£®
¢ŁŠ“³öŗģĮ×Č¼ÉյĻÆѧ·½³ĢŹ½£ŗ ””£»
¢ŚÄÜĖµĆ÷æÕĘųŹĒ»ģŗĻĪļµÄĻÖĻóĪŖ£ŗŗģĮ×Č¼ÉÕ½įŹųŗó£¬ĄäČ“µ½ŹŅĪĀ£¬“ņæŖÖ¹Ė®¼Š£¬”” £®
£Ø2£©Ķ¼BĖłŹ¾ŹµŃéæÉÓĆÓŚ²ā¶ØĖ®µÄ×é³É£®
¢Łµē½āĖ®·“Ó¦ÖŠÉś³ÉµÄĮ½ÖÖĪļÖŹŹĒ”” £»
¢Ś»¹ÄÜĖµĆ÷×é³ÉĖ®µÄŌŖĖŲÖÖĄąµÄŹµŃéŹĒ”” ””£®
ŹµŃ鶞£ŗĢ½¾æĪ¢Į£µÄŠŌÖŹ
ČēĶ¼CŗĶĶ¼DĖłŹ¾£¬ÓĆ¼×ŅŅĮ½Ö§“óŠ”ĻąĶ¬µÄ×¢ÉäĘ÷£¬·Ö±šĪüČ”µČĢå»żµÄæÕĘųŗĶĖ®£¬ÓĆŹÖÖø¶„×”×¢ÉäĘ÷Ä©¶ĖµÄŠ”æ×£¬ÓĆĻąĶ¬“󊔵ÄĮ¦£¬½«ĖØČūĀżĀżĶĘČė£®
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČŻŅ×±»Ń¹ĖõµÄ×¢ÉäĘ÷ŹĒ”” ””£ØĢī”°¼×”±»ņ”°ŅŅ”±£©
£Ø2£©Ķعż¶Ō±ČÉĻŹöĮ½øöŹµŃ飬æÉŅŌµĆ³ö£ŗŌŚĻąĶ¬Ģõ¼žĻĀ£¬æÕĘųµÄĪ¢Į£¼äµÄæÕĻ¶±ČĖ®µÄ £ØĢī”°“ó”±»ņ”°Š””±£©£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø7·Ö£©Ė®ŗĶČÜŅŗŌŚÉś²ś”¢Éś»īÖŠĘš×ÅŹ®·ÖÖŲŅŖµÄ×÷ÓĆ”£
£Ø1£©ĻĀĶ¼ŹĒµē½āĖ®ŹµŃé×°ÖĆ”£ŌŚŹµŃé¹ż³ĢÖŠ£¬ŹŌ¹Ü1²śÉśµÄĘųĢåŹĒ £¬Š“³öĖ®ŌŚĶصēĢõ¼žĻĀ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©½«Ō“Ė®“¦Ąķ³É×ŌĄ“Ė®µÄ¹ż³ĢÖŠŠč¼ÓČėÉśŹÆ»Ņ£¬ÉśŹÆ»ŅÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__ __”£
£Ø3£©20”ꏱ£¬ĀČ»ÆÄʵÄČܽā¶ČĪŖ36g£¬Ōņ20”ꏱĀČ»ÆÄʱ„ŗĶČÜŅŗÖŠČÜÖŹŗĶČܼĮµÄÖŹĮæ±ČĪŖ ”£
£Ø4£©ĪŖĮĖ½ųŠŠÅ©ŅµŃ”ÖÖ£¬ĻÖ½«200g30%µÄĀČ»ÆÄĘČÜŅŗĻ”ŹĶĪŖ10%µÄĀČ»ÆÄĘČÜŅŗ£¬ŠčŅŖ¼ÓĖ®µÄÖŹĮæĪŖ ”£
£Ø5£©×ŌĄ“Ė®ÖŠĶØČėÉŁĮæĀČĘųæÉŅŌɱ¾śĻū¶¾£¬»Æ¹¤³§³£ÓĆÅØ°±Ė®¼ģ²āĀČĘų“¢“ęÉč±ø»ņÕߏäĘų¹ÜŹĒ·ńÓŠĀČĘųŠ¹Ā©”£A”¢B”¢C”¢D±ķŹ¾4ÖÖĪļÖŹ£¬ĘäĪ¢¹ŪŹ¾ŅāĶ¼¼ūĻĀ±ķ£¬AŗĶBŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³ÉCŗĶD”£
| ĪļÖŹ | A | B | C | D |  |
| »ÆѧŹ½ | NH3 | Cl2 | N2 | | |
| Ī¢¹ŪŹ¾ŅāĶ¼ |  |  |  |  |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ė®ŌŚ»ÆѧŹµŃéÖŠµÄ×÷ÓĆ²»æÉŗöŹÓ”£ČēĶ¼ĖłŹ¾µÄĖÄøöŹµŃéÖŠ·Ö±šÓƵ½Ė®”£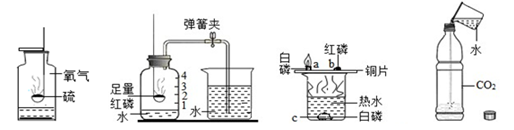
| A£®ĮņŌŚŃõĘųÖŠČ¼ÉÕ | B£®²ā¶ØæÕĘųÖŠŃõĘųŗ¬Įæ | C£®Ģ½¾æČ¼ÉÕĢõ¼ž | D£®Ģ½¾æ¶žŃõ»ÆĢ¼ŠŌÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©»ÆѧÓėÉś»īĆÜĒŠĻą¹Ų”£ĒėÓĆĻą¹ŲµÄ»ÆѧÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Ģś¹ųŹĒÓŠŅęÓŚČĖĄą½”æµµÄĄķĻė“¶¾ß£¬Ö÷ŅŖŌŅņŹĒ £¬µ«ĖüŗÜČŻŅ×øśĖ®ŗĶ
µČ·¢Éś·“Ó¦¶ųÉśŠā£¬Š“³öŅ»ÖÖŌ¤·ĄĢś¹ųÉśŠāµÄ¾ßĢå×ö·Ø ”£
£Ø2£©ĮŁ½üÖŠæ¼£¬ĀčĀčĪŖĮĖøųŠ”ĄöŌö¼ÓÓŖŃų£¬ÖʶØĮĖĪē²ĶŹ³Ę×ČēĻĀ£ŗĆ×·¹”¢Õؼ¦ĶČ”¢ĒåÕōÓć”¢Å£ÄĢ”£Ź³Ę×ÖŠø»ŗ¬ĢĒĄąµÄŹ³ĪļŹĒ £¬ĪŖĮĖÓŖŃų¾łŗā£¬Äć½ØŅ銔ĄöµÄĀčĀč»¹Ó¦Ōö¼ÓµÄŹ³ĪļŹĒ £ØŠ“Ņ»ÖÖ£©”£
£Ø3£©½ńğğ³õĪŅ¹ś¶ąµŲ³öĻÖĮĖĪķö²ĢģĘų£¬PM2.5ŹĒŌģ³ÉÕāÖÖĢģĘųµÄ”°ĪŽŠ×”±Ö®Ņ»”£
¢ŁPM2.5ŹĒÖø“óĘųÖŠÖ±¾¶Š”ÓŚ»ņµČÓŚ2.5Ī¢Ć×µÄæÅĮ£Īļ£¬Ņ²³ĘæÉČė·ĪæÅĮ£Īļ”£ĻĀĮŠŠŠĪŖ»į²śÉśPM2.5µÄŹĒ ”£
| A£®½«Š£Ō°µÄĄ¬»ų¶Ń»żĘšĄ“£¬¾ĶµŲ·ŁÉÕ | B£®ĪŖ·½±ć³öŠŠ£¬Ģį³«“óĮæ¹ŗĀņŗĶŹ¹ÓĆĖ½¼Ņ³µ |
| C£®“óĮ¦·¢Õ¹»šĮ¦·¢µē | D£®½ŚČÕĄļČ¼·Å±ŽÅŚ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(6·Ö)Ė®ŹĒ×īĘÕĶØ”¢×ī³£¼ūµÄĪļÖŹÖ®Ņ»”£
£Ø1£©³¤ĘŚŹĒŅūÓĆÓ²Ė®²»ĄūÓŚ½”æµ£¬Ó²Ė®ŗĶČķĖ®µÄĒų±šŹĒ_______________________”£
£Ø2£©µē½āĖ®æÉÖ¤Ć÷Ė®ÓÉĒā”¢ŃõĮ½ÖÖŌŖĖŲ×é³É£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©Ė®ŹĒÖŲŅŖµÄČܼĮŗĶ»Æ¹¤ŌĮĻ”£ĀČ¼ī¹¤ŅµŅŌ±„ŗĶŹ³ŃĪĖ®ĪŖŌĮĻ»ńµĆÉÕ¼īµČ»Æ¹¤²śĘ·£¬·“Ó¦ŌĄķĪŖ:2NaCl+2H2OĶصē2NaOH+H2”ü+Cl2”ü”£
¢Ł 20”ꏱ£¬NaClµÄČܽā¶ČŹĒ36g”£øĆĪĀ¶ČĻĀ£¬±„ŗĶŹ³ŃĪĖ®ÖŠČÜÖŹÓėČܼĮµÄÖŹĮæ±ČĪŖ ”£
¢Ś ÉÕ¼īæÉÓĆÓŚ“¦ĄķĮņĖįŠ¹Ā©£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©Ė®ŌŚ»ÆѧŹµŃéÖŠ¾ßÓŠÖŲŅŖ×÷ÓĆ”£½«ĢśĖæ·ÅŌŚ
³±ŹŖµÄæÕĘųÖŠ£ØČēĶ¼ĖłŹ¾£©£¬Ņ»¶ĪŹ±¼äŗó£¬
¹Ū²ģµ½µ¼¹ÜÄŚŅŗĆęÉĻÉż;“ņæŖK£¬µĪ¼ÓĻ”ŃĪĖį£¬
¹Ū²ģµ½µÄĻÖĻóŹĒ ”£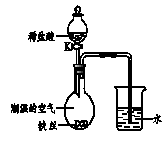
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
2013 Äź¼ĆÄĻŹŠÕūŗĻõĄĶ»ČŖ”¢“óĆ÷ŗž”¢ĪåĮśĢ¶”¢»¤³ĒŗÓµČĀĆÓĪ׏Ō“£¬×é½ØĢģĻĀµŚŅ»ČŖ·ē¾°Ēų£¬Ėü¼Æ¶ĄĢŲµÄ×ŌČ»É½Ė®¾°¹ŪŗĶÉīŗńµÄĄśŹ·ĪĻƵ×ŌĢÓŚŅ»Ģ壬ĪŖ 5A ¼¶¾°Ēų”£
£Ø1£©õĄĶ»ČŖĖ®ŹōÓŚ__________£ØĢī”°“æ¾»Īļ”±»ņ”°»ģŗĻĪļ”±£©”£
£Ø2£©Ä³»ÆѧŠ”×éµÄĶ¬Ń§¶Ō»¤³ĒŗÓµÄĖ®Ńł½ųŠŠĮĖĻą¹ŲµÄŃŠ¾æ£¬ĻÖŅŖÓĆ pH ŹŌÖ½“ÖĀŌ²ā¶ØŗÓĖ®µÄĖį¼īŠŌĒæČõ£¬²ā¶ØµÄ¾ßĢå·½·ØŹĒ
£Ø3£©ĪŖĮĖ¼ģŃé“óĆ÷ŗžĖ®ŹĒČķĖ®»¹ŹĒÓ²Ė®£¬æÉĻņĖ®ŃłÖŠ¼ÓČė___________£¬½Į°č”£
£Ø4£©¼ĆÄĻŹĒČŖĖ®Ö®¶¼£¬¶ŌČŖĖ®µÄĻĀĮŠĖµ·ØÖŠ“ķĪóµÄŹĒ £ØĢīŠņŗÅ£©
| A£®Ēå³ŗµÄČŖĖ®ŹĒČÜŅŗ | B£®ÕōĮóŹĒ¾»»Æ³Ģ¶Č×īøߵľ»Ė®·½·Ø |
| C£®æÉÓĆÖ󷊵ķ½·Ø½µµĶČŖĖ®µÄÓ²¶Č | D£®¹żĀĖæÉŅŌ³żČ„ČŖĖ®ÖŠµÄæÉČÜŠŌŌÓÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©Ė®ŹĒŅ»ĒŠÉśĪļÉś“ęĖł±ŲŠčµÄ£¬ĪŅĆĒÓ¦øĆĮĖ½āÓŠ¹ŲĖ®µÄŅ»Š©ÖŖŹ¶”£
£Ø1£©±£»¤Ė®»·¾³”¢Õä°®Ė®×ŹŌ“£¬ŹĒĆæøö¹«ĆńÓ¦¾”µÄŌšČĪŗĶŅåĪń”£ĻĀĮŠ×ö·ØÓŠĄūÓŚ±£»¤Ė®×ŹŌ“µÄŹĒ (ĢīŠņŗÅ)”£
A£®“óĮæŹ¹ÓĆ»Æ·ŹÅ©Ņ© B£®¹¤Ņµ·ĻĖ®“¦Ąķ“ļ±źŗóÅÅ·Å
C£®Ź¹ÓĆŗ¬Į×Ļ“ŅĀ·Ū D£®Éś»īĪŪĖ®Ö±½ÓÅÅ·Å
£Ø2£©Ė®Ņ²ŹĒ×ī³£¼ūµÄČܼĮ£¬½«ĻĀĮŠĪļÖŹ¼ÓČė×ćĮæµÄĖ®ÖŠ£¬ÄÜŠĪ³ÉĪŽÉ«ČÜŅŗµÄŹĒ ”££ØĢī×ÖÄøŠņŗÅ£©
A£®Ö²ĪļÓĶ B£®ÕįĢĒ C£®CaCO3 D£®øßĆĢĖį¼Ų
£Ø3£©ČܽāĮĖ½Ļ¶ąµÄµÄæÉČÜŠŌøĘŗĶĆ¾µÄ»ÆŗĻĪļµÄĖ®ŹōÓŚÓ²Ė®”£Ó²Ė®¶ŌÉś²ś”¢Éś»ī¶¼ÓŠ²»Į¼Ó°Ļģ”£Éś»īÖŠ£¬Ņ»°ćæÉÓĆ µÄ·½·ØĄ“Ź¹Ó²Ė®×Ŗ»ÆĪŖČķĖ®”£
£Ø4£©ĪŅ¹śŃŠÖĘ³ö±ČĘÆ°×·Ūøüøߊ§µÄŅūÓĆĖ®Ļū¶¾¼Į”°ClO2”±£¬ÖĘČ”ClO2·“Ó¦ĪŖ£ŗ
X + 2NaClO2 ="=" 2ClO2 + 2NaCl£¬ŌņXµÄ»ÆѧŹ½ĪŖ ”£
£Ø5£©Š“³öŅ»øöÓŠĖ®²Ī¼ÓµÄ»ÆŗĻ·“Ó¦µÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ ”£
£Ø6£©ŌŚŹµŃéŹŅÓĆÅØĮņĖįÅäÖĘĻ”ĮņĖįŹ±£¬ŠčŅŖÓƵ½Ė®£¬ĘäÖ÷ŅŖ²½ÖčÓŠ£ŗ¼ĘĖć”¢ ”¢»ģŌČ”¢ĄäČ“ÖĮŹŅĪĀ×°Ęæ²¢ĢłÉĻ±źĒ©”£ ČōŅŖÅäÖĘ184gÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ĮņĖį£¬ŠčŅŖÖŹĮæ·ÖŹżĪŖ98%µÄÅØĮņĖį(ĆܶČĪŖ1.84 g/cm3) mL(¼ĘĖć½į¹ū±£ĮōŅ»Ī»Š”Źż)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°“ŅŖĒ󊓳ö»Æѧ·½³ĢŹ½²¢»Ų“šÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©Ė®ŌŚĶصēŹ±·¢Éśµē½ā£ŗ””_ ________”””£
ŌŚøĆŹµŃéÖŠ£¬æÉ擵½Õż¼«Óėøŗ¼«Į½ĘųĢåµÄĢå»ż±ČŹĒ””_________””£»øĆŹµŃéµĆ³öµÄ½įĀŪŹĒĖ®ÓÉ””____ _____×é³É”£
£Ø2£©ĻøĢśĖæŌŚ“æŃõĘųÖŠČ¼ÉÕ£ŗ””__ _______”””£
ŌŚŹµŃéÖŠŅŖĻņ¼ÆĘųĘæÖŠŌ¤ĻČ·ÅÉŁĮæµÄĖ®»ņÕßĻøɳ£¬ŌŅņŹĒ””___ __ ____ ”””£
£Ø3£©ĻĀĶ¼¼×Ķ¼ÖŠŗģĮ×Č¼ÉյĻÆѧ·½³ĢŹ½ĪŖ””_______ __ ”””£
“ņæŖÖ¹Ė®¼Šŗó擵½µÄĻÖĻóŹĒ””_______ __£¬ĖµĆ÷æÕĘųÖŠŃõĘų“óŌ¼Õ¼_ ___£¬×īĻČĶعżŹµŃéµĆ³öÕāøö½įĀŪµÄ»Æѧ¼ŅŹĒ__ __£ØĢīŠņŗÅ£©”£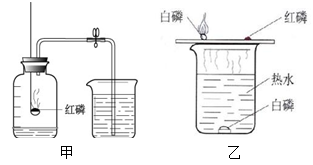
| A£®ĄĶßĪż | B£®ÕÅĒąĮ« | C£®µĄ¶ū¶Ł | D£®ĢĄÄ·Éś |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com