�⣺��1��������ֶ������ĺϽ��Ǵ����ﵱȻ���ǵ��ʣ����ǵ���ȵĵ��壻�ڿ���������������ˮ��Ӧ�����⣬������������ȼ������������������
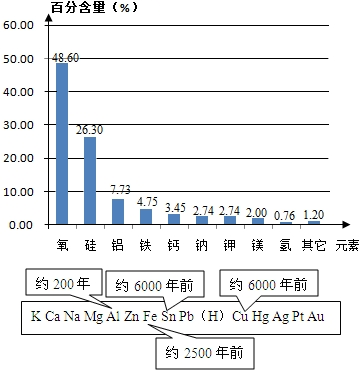
��2���ؿ���Ԫ�صĺ�������ǰ��λ��Ԫ�طֱ��ǣ������衢�����������Ժ������Ľ���������
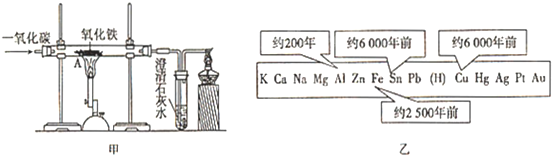
��3��������Һ�к�������ͭ���������Ļ�Ա�ͭǿ����������ͭ������ͭ��Һ���û���������˲���������Ʒ���Ʋ�����Һ����صķ���ʽΪ��Fe+CuSO
4�TFeSO
4+Cu��
��4����������ʵ��������������е�������ˮ��ͬ���õĽ����
��5�����Ļ�ѧ���ʽϻ����ڿ���������������Ӧ�����������������������������棬���˱������Ľ����������ã�
��6��������Fe
2O
3����Ԫ�ص���������=

��100%=70%
1000t��������80%�ij�����ʯ�У�������������=1000t��80%=800t
�����Ͽ�������������4%������������=
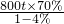
��583.3t
��7������������������Ϊx��
Zn+H
2SO
4=ZnSO
4+H
2��
65 2
6.5g x

=
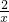
x=0.2g
��1��A ��2��Al����������3��Fe+CuSO
4�TFeSO
4+Cu����4�������е�������ˮ��5������������Ӧ���������ܵ���������Ĥ�������������棬���������Ľ���������6��583.3��7��0.2
��������1����������������ʷ����жϣ�
��2�����ݵؿ���Ԫ�ص������������жϣ�
��3�����ݽ������˳���л��ǿ�Ľ������ѻ�����Ľ�����������Һ���û������Ĺ��ɷ����жϣ�
��4�������������ԭ�������жϣ�
��5����������������Ӧ�Ļ�ѧ���ʷ����жϣ�
��6���ݻ�ѧ�仯ǰ��Ԫ���������䣬������������4%����������Ԫ��������1000t��������80%�ij�����ʯ��������Ԫ��������ȣ�������������������
��7����п����������п�����ᷴӦ�Ļ�ѧ����ʽ���Լ��������������������
������������һ����������صĿ����⣬�漰����������������������ʣ�ֻ�жԳ��������������������գ��������ɽ��⣮

 ��100%=70%
��100%=70%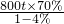 ��583.3t
��583.3t =
=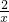


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�