
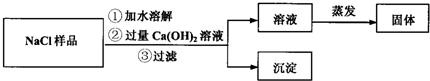
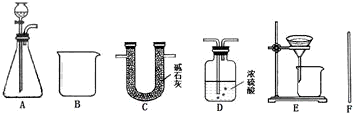
| ���� | ҩƷ | ʵװ�� | �������� ����ag��bg��cg��dg��ʾ�� | Na2CO3��������������ʽ |
| һ | ________ | D��E��F | ________ | ________ |
| �� | ________ | ________ | ag��cg��dg | ________ |
| �� | ________ | ________ | ________ | ________ |
| ���� | ҩƷ | ʵװ�� | �������� ����ag��bg��cg��dg��ʾ�� | |
| һ | CaCl2��Һ | D��E��F | ag��bg |  % % |
| �� | ϡH2SO4 | A��D��C | ag��cg��dg | 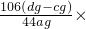 100% 100% |
| �� | �� ϡH2SO4 | ��A | ag��cg��dg | 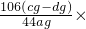 100% 100% |
| ���� | ҩƷ | ʵװ�� | �������� ����ag��bg��cg��dg��ʾ�� | |
| һ | CaCl2��Һ | D��E��F | ag��bg |  % % |
| �� | ϡH2SO4 | A��D��C | ag��cg��dg | 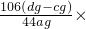 100% 100% |
| �� | �� ϡH2SO4 | ��A | ag��cg��dg | 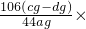 100% 100% |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������һ����顡���꼶��ѧ��(��) �˽̰� ���ͣ�058
| |||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
30����7�֣�ijУ��һ�ó��л�ѧ�Σ����ȣ���ʦ��һֻ�����С�ձ��зż����������ƹ��壬��ͬѧ�ǹ۲졣���ְ�ɫ���������ƹ���ܿ����䳱��ճ��һ��Ȼ����ʦҪ��ѧ���Լ���������100g2.5%������������Һ�����м�ͬѧ��ʵ��������£�
��1����ȡ2.5g�����������ƣ�����ƽ�����˸���һ��������ͬ��ֽ��������ƽ��㣬Ȼ�������̷�2g���룬��������0.5g���������̼��������ƹ�������ƽƽ�⡣
��2��������Һ������Ͳ��ȡ100mLˮ��Ȼ���������Ƽ�����Ͳ�У��ӱ߽��衣��ʦ�Լ�ͬѧ������ָ������ͬѧ�������Լ������е��Ĵ�����
��________________________________________________��
��________________________________________________��
��________________________________________________��
��________________________________________________��
��3�������ʦ��ѧ�����ۣ��������3�ַ�����������һ�ַ�������ѧ�仯��������Ҫ�����ķ��룬Ҳ�������������ʣ���100g��2.5%������������Һ��Ϊ5%������������Һ��ͬѧ��չ�������ҵ����ۣ�������з������£�
________________________________________��
_______________________________________��
_______________________________________��
________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com