
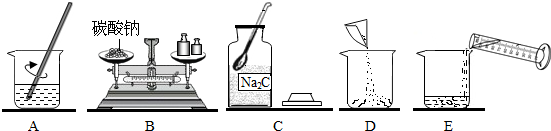
| ���� |
| �ܶ� |
| 80g |
| 1g/mL |
| 106 |
| 20%��53g |
| 44 |
| x |
| 106 |
| 20%��53g |
| 117 |
| y |
| 11.7g |
| 53g+68.4g-4.4g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | �����ֵ����� | ����һ | ������ |
| A | �����͵��� | ��������ǵ�Сľ�� | �۲�������ɫ |
| B | CO��CO2 | ͨ������ʯ��ˮ�й۲��Ƿ����� | ͨ��ˮ�й۲��Ƿ��� |
| C | ˮ�;ƾ� | ����ζ | Ʒ��ζ�� |
| D | ̼狀���� | ����ζ | �۲���ɫ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
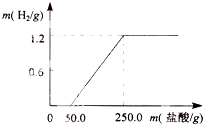 ij��ȤС��ӷ������ײ���һ����Ƭ����������21.9%��ϡ�����У������������������������������������ͼ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�
ij��ȤС��ӷ������ײ���һ����Ƭ����������21.9%��ϡ�����У������������������������������������ͼ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
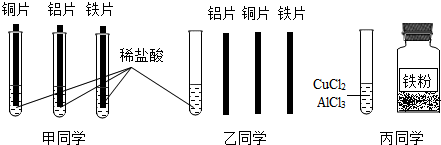
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �������� | ���������� | �������� | ������ | ���� |
| ��ɫ | ��ɫ | ��ɫ | ��ɫ | ��ɫ |
| �ܷ������� | �� | ���� | ���� | �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com