
����ͭ��Һ��H2O2�ķֽ��д����ã���ͬѧ��������������Һ��Ҳ���������Ӧ����ͬ�������ã����������������µ�̽����
����������������ʵ�鱨��
| ʵ����� | ʵ������ | ʵ����� |
| ��һ֧�Թ��м���5ml��H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թ��� | FeCl3��Һ���Դ��ֽ�H2O2 |
����֪FeCl3��ˮ�пɷ����Fe3+��Cl��,ͬѧ��������²���
�ټIJ��룺�������ֽ��H2O2����FeCl3��Һ��Fe3+
���ҵIJ��룺�������ֽ��H2O2����FeCl3��Һ��Cl��
�۱��IJ��룺�������ֽ��H2O2����FeCl3��Һ��H2O
����Ϊ����ܵ��� �IJ��룬������ ��
ͬѧ�Ƕ����µ��������룬��ʵ�����̽����������������
| ʵ����� | ʵ������ | ʵ����� |
| ��ʢ��5ml��5% ��H2O2��Һ���Թ��м������������ᣬ���Ѵ����ǵ�ľ�������Թ��� | �����Ե����� | |
| ��ʢ��5ml��5% ��H2O2��Һ���Թ��м��������� ��������ǵ�ľ�� |
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ���� | ʵ����� | ʵ������ |
�� |
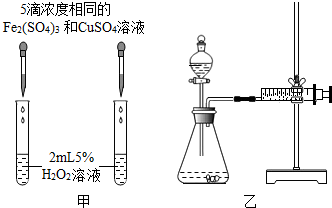
�ֱ����Թ�A��B�м��� 5mL 5%����ҺŨ�ȣ� H2O2��Һ��������2 ����ͬŨ�ȵ�CuSO4��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݣ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5mL 5%H2O2��Һ��5mL 10%H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ��Һ��H2O2�ķֽ��д����ã���ͬѧ��������������Һ��Ҳ���������Ӧ����ͬ�������ã����������������µ�̽����
����������������ʵ�鱨��
| ʵ����� | ʵ������ | ʵ����� |
| ��һ֧�Թ��м���5ml��H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թ��� | FeCl3�� |
����֪FeCl3��ˮ�пɷ����Fe3+��Cl—,ͬѧ�� ������²���
������²���
�ټIJ��룺�������ֽ��H2O2����FeCl3��Һ��Fe3+
���ҵIJ��룺�������ֽ��H2O2����FeCl3��Һ��Cl—
�۱��IJ��룺�������ֽ��H2O2����FeCl3��Һ��H2O
����Ϊ����ܵ��� �IJ��룬������ ��
ͬѧ�Ƕ����µ��������룬��ʵ�����̽����������������
| ʵ����� | ʵ������ | ʵ����� |
| ��ʢ��5ml��5% ��H2O2��Һ���Թ� | �����Ե����� | |
| ��ʢ��5ml��5% ��H2O2��Һ���Թ��м��������� ��������ǵ�ľ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���������п���ѧ�Ծ���A�����������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com