
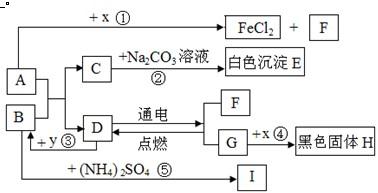
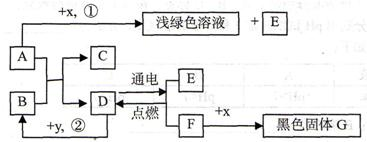
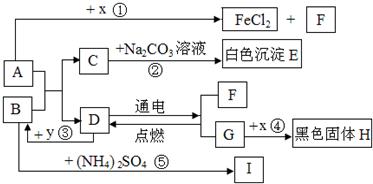
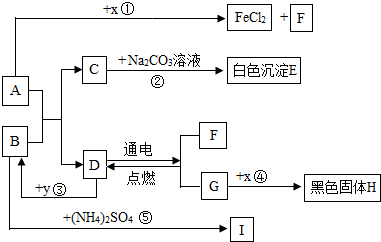
A”¢BæÉ·¢ÉśÖŠŗĶ·“Ó¦£¬DŹĒÉś»īÖŠ×ī³£¼ūµÄŅŗĢ唣Ķس£ĒéæöĻĀ£¬F”¢G”¢IĪŖĘųĢ壬ĒŅFŹĒŅ»ÖÖĒå½ąÄÜŌ“”£![]() ŹĒÄæĒ°Ó¦ÓĆ×ī¹ć·ŗµÄ½šŹō£¬
ŹĒÄæĒ°Ó¦ÓĆ×ī¹ć·ŗµÄ½šŹō£¬![]() ³£ÓĆ×÷Ź³Ę·øÉŌļ¼Į”£ø÷ĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Øøö±š²śĪļĀŌČ„£©”£
³£ÓĆ×÷Ź³Ę·øÉŌļ¼Į”£ø÷ĪļÖŹµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Øøö±š²śĪļĀŌČ„£©”£
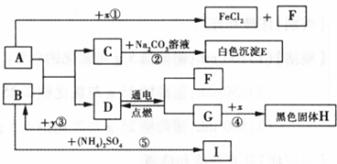
£Ø1£©BĖ×³Ę ”£
£Ø2£©FÓėG»ģŗĻŗóÓöĆ÷»šæÉÄÜ·¢Éś £¬ÓėF“ĖŠŌÖŹĻąĖʵÄĘųĢ廹ӊ ”£
£Ø3£©·“Ó¦¢ŁµÄ·“Ó¦ĄąŠĶŹĒ £»Ź¹ÓĆ½šŹō![]() ÖĘ³ÉµÄµ¶¾ßĒŠ”¢Ļ÷²¤ĀܵČĖįŠŌĖ®¹ūŗóÓ¦¼°Ź± £¬ŌŅņŹĒ ”£
ÖĘ³ÉµÄµ¶¾ßĒŠ”¢Ļ÷²¤ĀܵČĖįŠŌĖ®¹ūŗóÓ¦¼°Ź± £¬ŌŅņŹĒ ”£
£Ø4£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø5£©·“Ó¦¢ŻÖŠ£¬½«BµÄ¹ĢĢå·ŪÄ©Óė£ØNH4£©2SO4µČ»ģŗĻŃŠÄ„£¬æÉĒų±š ·ŹÓė¼Ų·Ź”££Ø6£©·“Ó¦¢Ū·¢ÉśŹ±·Å³ö“óĮæČČ£¬»Æѧ·½³ĢŹ½ĪŖ ”£·“Ó¦¢ÜµÄ·“Ó¦Ģõ¼žŹĒ £¬ŹµŃéĻÖĻóŹĒ £¬»ÆѧŹĒŃŠ¾æĪļÖŹ¼°Ęä±ä»ÆµÄæĘѧ”£±Č½Ļ·“Ó¦¢Ū”¢¢Ü£¬Äć¶Ō»Æѧ±ä»ÆµÄČĻŹ¶ŹĒ£ŗ£ØŠ“2Ģõ£© ”¢ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com