
·ÖĪö £Ø1£©øł¾Ż»ģŗĻĪļ֊ijĪļÖŹµÄÖŹĮæ=»ģŗĻĪļµÄÖŹĮæ”ĮøĆĪļÖŹµÄÖŹĮæ·ÖŹż£¬»ÆŗĻĪļµÄÖŹĮæ=øĆ»ÆŗĻĪļ֊ijŌŖĖŲµÄÖŹĮæ”ĀøĆŌŖĖŲµÄÖŹĮæ·ÖŹż£¬½ųŠŠ·ÖĪö½ā“š£®
£Ø2£©øł¾ŻMgSO4”¢MgSO3µÄ»ÆѧŹ½£¬æÉŅŌ·¢ĻÖĖüĆĒµÄ1øö·Ö×ÓÖŠ¾łŗ¬ÓŠ1øöĆ¾Ō×ÓŗĶ1øöĮņŌ×Ó£¬æɼĘĖć³öĮ½ÖÖŌŖĖŲµÄÖŹĮæ±Č£¬¾ĶæÉŅŌĶعżĮņµÄÖŹĮæ·ÖŹżĒóĆ¾µÄÖŹĮæ·ÖŹż£¬ÓąĻĀµÄ¾ĶŹĒŃõŌŖĖŲÖŹĮæ·ÖŹż£®
£Ø3£©øł¾Ż»ÆŗĻĪļµÄÖŹĮæ=øĆ»ÆŗĻĪļ֊ijŌŖĖŲµÄÖŹĮæ”ĀøĆŌŖĖŲµÄÖŹĮæ·ÖŹż£¬½ųŠŠ·ÖĪö½ā“š£®
½ā“š ½ā£ŗ£Ø1£©ĻÖÓŠ100tŗ¬Fe2O3ÖŹĮæ·ÖŹżĪŖ80%µÄ³ąĢśæóŹÆ£ØŌÓÖŹÖŠ²»ŗ¬ĢśŌŖĖŲ£©£¬øĆ³ąĢśæóŹÆĖłŗ¬ĢśŌŖĖŲµÄÖŹĮæĪŖ100t”Į80%”Į$\frac{56”Į2}{56”Į2+16”Į3}$”Į100%=56t£®
£Ø2£©MgSO4”¢MgSO3µÄ»ÆѧŹ½£¬æÉŅŌ·¢ĻÖĖüĆĒµÄ1øö·Ö×ÓÖŠ¾łŗ¬ÓŠ1øöĆ¾Ō×ÓŗĶ1øöĮņŌ×Ó£¬Ōņ»ģŗĻĪļÖŠĆ¾ŌŖĖŲŗĶĮņŌŖĖŲµÄÖŹĮæ±ČĪŖ24£ŗ32=2£ŗ3£¬»ģŗĻĪļÖŠĮņŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ28%£¬ŌņĆ¾ŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ28%”Į$\frac{2}{3}$”Ö18.7%£¬ŌņŃõŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ1-28%-18.7%=53.3%£®
£Ø3£©£¬²āµĆ»Æ·ŹÖŠµŖŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ14.0%£¬ŌņøĆ»Æ·ŹÖŠĻõĖįļ§µÄÖŹĮæ·ÖŹżĪŖ14.0%”Ā£Ø$\frac{14”Į2}{14”Į2+1”Į4+16”Į3}$”Į100%£©=40%£®
¹Ź“š°øĪŖ£ŗ£Ø1£©56£»£Ø2£©53.3%£»£Ø3£©40%£®
µćĘĄ ±¾ĢāÄŃ¶Č²»“ó£¬æ¼²éĶ¬Ń§ĆĒ½įŗĻŠĀŠÅĻ¢”¢Įé»īŌĖÓĆ»ÆѧŹ½µÄŗ¬ŅåÓėÓŠ¹Ų¼ĘĖć½ųŠŠ·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāµÄÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĘųĢåæÉŃ¹Ėõ“¢“ęÓŚøÖĘæÖŠ----·Ö×ÓĢå»ż±äŠ” | |
| B£® | Ē½ÄŚæŖ»Ø£¬Ē½ĶāæÉĪŵ½»ØĻć-------·Ö×ÓŌŚ²»¶ĻŌĖ¶Æ | |
| C£® | Ė®Õō·¢ĪŖĖ®ÕōĘų£¬Ė®µÄ»ÆѧŠŌ֏ƻӊøıä-----Ķ¬ÖÖĪļÖŹµÄ·Ö×ÓŠŌÖŹĻąĶ¬ | |
| D£® | 1µĪĖ®“óŌ¼ÓŠ1.67¢Ŗ1021øöĖ®·Ö×Ó£¬·Ö×ÓµÄÖŹĮæŗĶĢå»ż¶¼ŗÜŠ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗģĮ×Č¼ÉÕ£¬²śÉś“óĮæ°×É«ŃĢĪķ | |
| B£® | ĢśĖæŌŚŃõĘųÖŠČ¼ÉÕ£¬»šŠĒĖÄÉä£¬Éś³ÉŗŚÉ«¹ĢĢå | |
| C£® | ľĢæŌŚŃõĘųÖŠČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼ | |
| D£® | ĮņŌŚŃõĘųÖŠČ¼ÉÕ»į²śÉśĆ÷ĮĮµÄĄ¶×ĻÉ«µÄ¹ā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¶žŃõ»ÆĮņ | B£® | ¶žŃõ»ÆĢ¼ | C£® | ĪåŃõ»Æ¶žĮ× | D£® | ĖÄŃõ»ÆČżĢś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
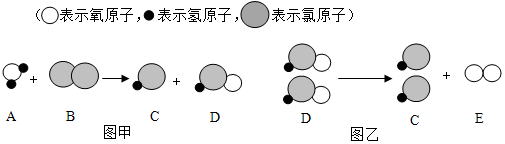
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | O2 | B£® | O | C£® | O2- | D£® | 2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com