
| 106 |
| x |
| 100 |
| 10.00g |
| 10.6g |
| 13.25g |
| 106 |
| x |
| 100 |
| 10.00g |
| 10.6g |
| 13.25g |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��SO2 |
| B��NO2 |
| C��N2 |
| D��NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ڿ����о���ȼ�գ���������ɫ���� |
| B�������ڿ����о���ȼ�գ������������� |
| C�������ڴ�����ȼ�գ��������䣬������ɫ����С���� |
| D��һ����̼ȼ���ܲ���ʹ����ʯ��ˮ����ǵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
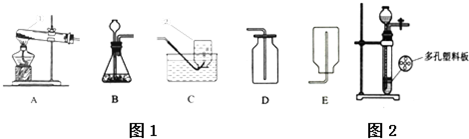
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ�� ���� | �ձ���ϡ��������� | ʯ��ʯ��Ʒ������ | �ձ������л��������� |
| 150g | 15g | 160.6g | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
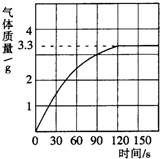 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ��������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������ϡ������뵽10gˮ���У�����CO2������������ͼ��ʾ������֪��ˮ������Ҫ�ɷ���̼��ƺ�������þ�����߶��������ᷴӦ����������þ�����ᷴӦ����������壩
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ��������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������ϡ������뵽10gˮ���У�����CO2������������ͼ��ʾ������֪��ˮ������Ҫ�ɷ���̼��ƺ�������þ�����߶��������ᷴӦ����������þ�����ᷴӦ����������壩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com