
£Ø12·Ö£©½įŗĻĶ¼Ź¾×°ÖĆ»Ų“šĻĀĮŠĪŹĢā”£
 |
A B C D E
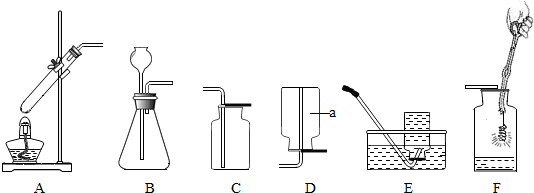
¢ÅŠ“³ö±źŗÅŅĒĘ÷µÄĆū³Ę£ŗ¢Ł_____________£¬¢Ś_____________£¬¢Ū_____________£»
¢ĘŹµŃéŹŅČōÓĆ¹żŃõ»ÆĒāČÜŅŗŗĶ¶žŃõ»ÆĆĢ»ģŗĻÖĘČ”ŃõĘų£¬ŌņÄćŃ”ÓƵķ¢Éś×°ÖĆŹĒ £¬Čē¹ūŅŖŹÕ¼Æ±Č½ĻøÉŌļµÄŃõĘų£¬Äć»įÓĆ ŹÕ¼Æ”£
¢ĒÓĆøßĆĢĖį¼ŲÖĘČ”ŃõĘųµÄ»ÆѧŹ½±ķ“ļŹ½ĪŖ ¢Ł £¬ČōŃ”ŌńAŗĶD×°ÖĆÖĘČ”²¢ŹÕ¼ÆŃõĘų£¬ŌņŅŖ¶ŌA×°ÖĆ½ųŠŠµÄøĽųŹĒ ¢Ś £¬
¢Ū ”£ŹµŃé½įŹųŹ±Ó¦ĻČ___________¢Ü___________ŌŁ____________¢Ż__________£¬·ńŌņ»įµ¼ÖĀ____________¢Ž__________ÕØĮŃŹŌ¹Ü”£
¢Č³£ĪĀĻĀŹµŃéŹŅæÉÓĆ½šŹōŠæĮ£ŗĶĻ”ĮņĖįŅŗĢå·“Ó¦ÖĘČ”½Ļ“æ¾»µÄĒāĘų£¬ŌņӦєŌńµÄ×°ÖĆ×éŗĻŹĒ________”£
 Ö„ĀéæŖ»ØæĪ³ĢŠĀĢåŃéĻµĮŠ“š°ø
Ö„ĀéæŖ»ØæĪ³ĢŠĀĢåŃéĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ¼ÓČČ |
| ¼ÓČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźĢģ½ņŹŠ±¦ŪęĒųÖŠæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com