������pHС��5.6�Ľ�ˮ���ҹ��ֵ�������������Ҫ���ɾ������ȼ�պ����ú�Լ�ijЩ�����������������ŷŵĶ����������壬����һϵ�л�ѧ��Ӧ���γɵģ�

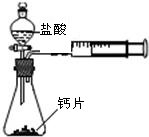
��1����������������ȼ�գ��۲쵽��������
����ȼ�գ��ų���������������������ɫ���棬����һ����ɫ�д̼�����ζ������
����ȼ�գ��ų���������������������ɫ���棬����һ����ɫ�д̼�����ζ������
���÷�Ӧ�Ļ�ѧ����ʽΪ
��
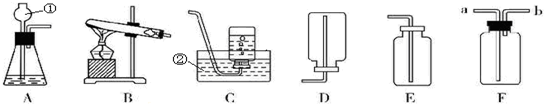
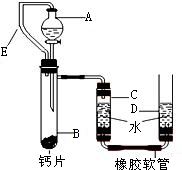
��2����λͬѧΪ��̽��ľ̿���Ƿ���������Ԫ�أ������������ͼ1��ʾʵ����вⶨ����д��ͼ������a�����ƣ�
����ǯ
����ǯ
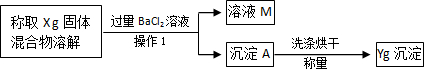
�����Dz������Ϻ��֪��������������ʹK
2Cr
2O
7��Һ��ɫ���ɳȺ�ɫ�����ɫ������Ӧ����ʽΪ����3SO
2+K
2Cr
2O
7+H
2SO
4�TK
2SO
4+

+H
2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��
Cr2��SO4��3
Cr2��SO4��3
����ӦǰK
2Cr
2O
7��CrԪ�ػ��ϼ�Ϊ
+6
+6
�ۣ�
��3����ͼ2��1994���2004��ij���е�һ�����ʱ�̲�Ŀ����ж�������ĺ���������˵������ȷ����
D
D
A��������ʾ��������еĶ����������������
B��������ʾ��1994��һ���д�Լ8�����Ҷ�������ĺ����ϸ�
C����������ĺ�����10��併�͵�ԭ������Ǽ�ǿ��ȼ�ϵ�����Ϳ����˶���������ŷ�
D������������Ⱦ����Ҫ��Դ�������ŷŵ�β����������Ⱦ�ķ����ǽ�ֹʹ������
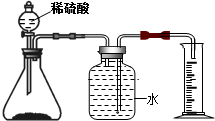
��4�����о�����Σ���Ĺ����У��ⶨ�õ�����ˮ��pH��ʵ�����
�ò�����պȡ��ˮ��������ֽ�ϣ������ɫ�����գ���ȡ��ֵ
�ò�����պȡ��ˮ��������ֽ�ϣ������ɫ�����գ���ȡ��ֵ
��
ij��ѧ��ȤС��ȡ�ս����������ˮ��ÿ��һ��ʱ���ýϾ��ܵ�pH�Ʋⶨ��pH���������£�
| �ⶨʱ��/���� |
0 |
1 |
2 |
3 |
| pH |
4.73 |
4.62 |
4.56 |
4.55 |
�ɴ˿�֪����ˮ������Խ��Խ
ǿ
ǿ
���ǿ���������������������ȶ������ܵ�ԭ������ˮ�е�H
2SO
3����������ΪH
2SO
4����ѧ����ʽΪ
2H2SO3+O2=2H2SO4
2H2SO3+O2=2H2SO4
��
��5��������ɵ�Σ���࣮ܶij��ȤС����鷢��ij�е�ʯ�̵ı����в�ͬ�̶ȵĸ�ʴ���ҽ�20��ĸ�ʴ�ٶȴ����������������Ҫԭ��֮һ�������꣮Ϊ�˼���ʯ�̸�ʴ��������һ�����飺
ʹ�������Դ
ʹ�������Դ
�������ʹ�����������ữ����ͼ3��ij��ȥ��ij����һ���в�ͬʱ�ں�ˮ��ƽ��pH�仯������ͼ����ȥ���ˮ������ǿ�ļ����ǵ�
��
��
���ȣ��ü�����ˮ������ǿ��ԭ������ǣ�����������
����ȡů����Ҫȼ�մ�����ʯȼ�ϣ��Ӵ��˶Ժ�ˮ����Ⱦ
����ȡů����Ҫȼ�մ�����ʯȼ�ϣ��Ӵ��˶Ժ�ˮ����Ⱦ
��
������ˮ�����٣���ˮ�����Ũ������
������ˮ�����٣���ˮ�����Ũ������
��
���껹��ʹ�����ữ��Ϊ���к���������������ʹ����ʯ�ҷ�ĩ����������ijɷ������ᣬ��д�����кͷ�Ӧ�Ļ�ѧ����ʽ
H2SO4+Ca��OH��2=CaSO4+2H2O
H2SO4+Ca��OH��2=CaSO4+2H2O
��
��6��ijУ��ѧ��ȤС����ѧ���Ļ�ѧ���ʺ��뵽��NaOH��Һ����SO
2����Ӧ��ѧ����ʽ���£�2NaOH+SO
2�TNa
2SO
3+H
2O ��NaOH��Һ����1000L�ѳ�ȥCO
2�Ŀ�����Ʒ����Һ����������0.64g����֪��ʱ�������ܶ�ԼΪ1.3g/L����
�ٱ����յ�SO
2������
0.8
0.8
g��
�ڷ�����Ӧ��NaOH�������������ԭ��������Na-23 S-32 O-16��
�ۿ�����SO
2��������������������ȷ��0.01%����





 +H2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��
+H2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��