
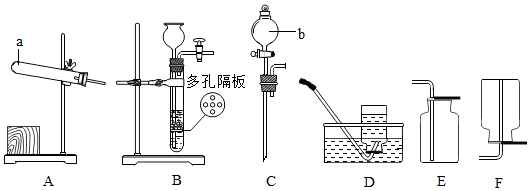
���� ��1������ʵ���ҳ������������ƺ�������ָ���������ý��з�����
��2�����ݸ�������ڼ��ȵ���������������ء��������̺������������ſ������ռ������������Һ©�����Կ���Һ��ĵμ��ٶȽ��з�����
��� �⣺��1��ͨ������������ָ���������ÿ�֪��a���Թܣ�b�Ƿ�Һ©����
��2����������ڼ��ȵ���������������ء��������̺���������ѧ����ʽΪ��2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2���������ſ������ռ��������������Ҫ�ռ�һƿ�����������Ӧѡ����ռ�װ����E����Һ©�����Կ���Һ��ĵμ��ٶȣ��������Ʊ�CO2����������IJ������ʣ�������Cװ�ã�
�ʴ�Ϊ����1���Թܣ���Һ©����
��2��2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2����E��C��
���� ������Ҫ���鳣������ķ���װ�����ռ�װ�õ�̽��������װ�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ���ռ�װ������������ܶȺ��ܽ���ѡ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ơ���ʯ�� | B�� | �������ơ���ʯ�� | C�� | ̼���ơ����� | D�� | ������̼��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
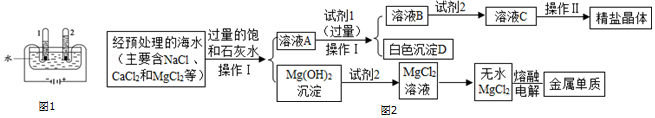
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������� | B�� | ��Ʒ��� | C�� | �ռ�֤�� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
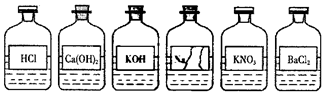
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ȡ�����Թ��� | �������ȷ ��صĻ�ѧ����ʽNa2CO3+2HCl=2NaCl+H2O+CO2�� ����Na2CO3+BaCl2=BaCO3��+2NaCl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
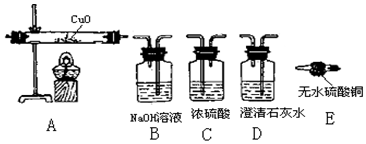
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��� | B�� | ��С | C�� | ���� | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com