
��8�֣��������ͼ��ա�
��1��д��ͼ�б�����������ƣ��� ��
��2��ʵ������װ��A��ȡ����ʱ������ҩƷ�� ���ѧʽ������Eװ���ռ�����������ʱ���� ��
��3��ʵ������Cװ����Ϊ��ȡ����ķ���װ�ã���Bװ����ȣ����Ե��ŵ��� ����C��Dװ����ȡ������̼���壬Cװ�õĵ��ܿ�Ӧ��Dװ�õĵ��ܿ� ���� ��a����b����������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��Dװ�û�������������;���罫�����л����������Ȼ��������ȥ������D��ʢ�� ��Һ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������ȡijЩ���������װ����ͼ��ʾ����ش��������⣺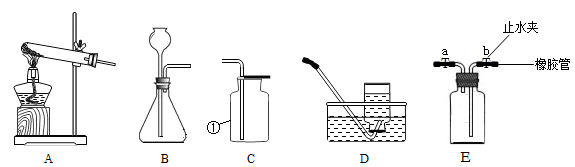
��1��ʵ�������ٵ������� ��
��2��ѡ��A��Dװ�ÿ���ȡ�������� ����Ӧ�Ļ�ѧ����ʽΪ ��
��3��ʵ������ȡ������̼����Ӧѡ��ķ���װ���� ����ѡ����ĸ������Ӧ�Ļ�ѧ����ʽΪ ��
��4������װ��E��ˮ�ռ�������ƿ����װ��ˮ������� (�a����b��)����ͨ
��ƿ�ڡ�����װ��E��ȥ�����л��е�ˮ������ƿ��Ӧʢ��������������
����дѡ���ţ���
A��Ũ���� B��ϡ���� C��ϡ���� D��ʯ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪʵ���ҳ�����һЩʵ��װ�ã������ͼ�ش��������⣺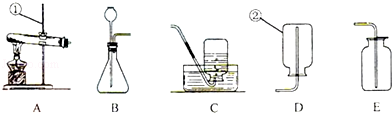
��1��д����ʵ�������١��ڵ����ƣ�����_________��������_________����
��2���ý���п��ϡ������ȡ����ʱ����ѡ�õ�ʵ��װ����_________��������ţ����䷴Ӧ�Ļ�ѧ����ʽ��_________����
��3���ø��������ȡ�������䷴Ӧ�Ļ�ѧ����ʽ����_________��������ѡ��Aװ�ò��ø������ΪҩƷ��ȡ��������Aװ�õĹܿڴ�Ӧ����_________������A��C���ӽ���ʵ�飬ʵ�����ʱ��Ӧ�Ƚ������Ƴ�ˮ��Ȼ����ֹͣ���ȣ���������Ŀ������_________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣���ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ����ѧ��ȡ�õķ�˶�ɹ�������ʵ�����Ҫ���÷ֲ����ġ��������ʵ��װ��ͼ�ش����⣺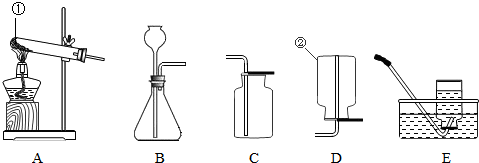
��1��д��ָ�����������ƣ��� ��
��2��ʵ��������ˮ�����ƹ��塢��ʯ�ҹ�����ȡ�ܶȱȿ���С��������ˮ�ļ��飬��ѡ�õķ���װ���� ��ѡ����ĸ����ͬ������ѡ�õ��ռ�װ���� ��
��3��ʵ���ҿ���B��Eװ����ȡ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)��ͼ������ʵ������ȡ����ij���װ�á�
��1���Ʊ�CO2Ӧѡ�õ�װ���� �����ţ�,����CO2�Ļ�ѧ����ʽΪ ��
��2��Bװ�õ������Լ�飺����ֹˮ�м�ס���ܵ���Ƥ�ܣ�Ȼ���� �м���ˮ���γ�һ��ˮ�������ã����۲쵽 ��˵��װ�ò�©����
��3������ƿ������ļ��飺������ƿ����� ����
�� ��˵����������CO2��
��4���ռ���һƿ������̼����ͼ��ʾ����ʵ�飬�۲쵽�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣���λͬѧ�������и�ʵ��װ��ͼ����ƺ��������������⣺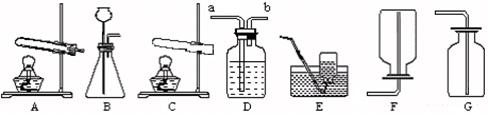
��1��ʵ��������װ��B������ȡCO2���壬��Ӧ����ʽ�� ��
��2��ʵ��������װ��A��E��������ȡ���ռ��������� ����ʵ���������ռ�����ֹͣʵ��ʱ����Ҫ�ر�ע��IJ����� ���������װ��D���ռ����壬������Ӧ���� ����дװ���еġ�a����b������
��3��װ��G�������ռ����壬�������ܶ�Ӧ�ȿ����أ������⣬��Ӧ�߱��������� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ȤС��������ͼװ�ý��ж�����̼����ȡ�Ͳ�������ʵ�顣��ش��������⣺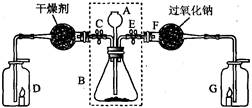
��1����д��ͼ�����������ƣ�A ��D ��
��2���ر�ֹˮ��E��ֹˮ��C����A�м���Һ������ƿB�еĹ���Ӵ������ж�����̼����������۲쵽D�е���������Ϩ��˵��������̼���� �� �Ļ�ѧ���ʡ���д��ʵ������ȡ������̼�Ļ�ѧ����ʽ�� ��
��3���ر�C��E����۲쵽G�е�����ȼ�ո�����˵��ͨ��G�е������� ��
��4����ȤС���Fװ���������ķ�Ӧ����̽����
[��������] ������̼����������Ʒ�Ӧ����̼���ƺ�ͨ��G�е����塣
[ʵ��̽��] ����װ��F�з�Ӧ��Ĺ������ʺ���̼���ơ�
�� ��F�з�Ӧ��Ĺ�����������ˮ�У������̪��Һ���۲쵽��Һ��ɺ�ɫ��˵���ù������ʵ�ˮ��Һ�� �ԡ�
�� �������Ϻ��֪�������ʵ���ɫ��ӦΪ��ɫ��֤��������Ԫ�ء�
�� ��������������֤��
| �� �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ��F�з�Ӧ��Ĺ�����������ˮ�У� a������������ �� | b�� | װ��F�з�Ӧ��Ĺ������ʺ���CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�ʵ���Ҳ���װ����ͼ��ʾ����ش��������⡣
��1������X�������� ��
��2��ʵ������CaCO3��ϡ�����Ʊ�CO2�Ļ�ѧ����ʽΪ �������Ʊ�22g CO2������ʵ����ģ�������Ҫ���� g CaCO3��
��3�����ô���ʯ��ϡ������ȡCO2ʱ������Ϊ����װ�õ��� �� ������ĸ����ͬ����
����Ҫ�Ʊ���ƿCO2��������ȡ�����б��ڲ���ϡ���ᣬ����װ��Ӧѡ�� ���ռ�װ��Ӧѡ�� ��
��4��ʵ����ͨ�������������ƺ��Ȼ�淋Ļ����Һ��ȡ���ռ�N2ʱ��Ӧѡ�� ��
��϶��ɵ�װ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣���ͼ��ʵ���ҳ�������ȡ�����װ�ã���ش�
��1����Aװ����ȡ����ʱ����Ӧ���״̬�ͷ�Ӧ����������ʲô������
��2��д����Bװ����ȡ�����Ļ�ѧ����ʽ �������������ļ��鷽����
��3��ʵ������ʯ��ʯ��ϡ�����ƵõĶ�����̼�������Ȼ����ˮ��������ѡ������װ����ȡ
���ռ���������Ķ�����̼��װ�õ���ȷ����˳��ΪB�� ��C����װ����ţ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com