
| ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ϡ��������� | 5g | 5g | 5g | 5g |
| ʣ���������� | 3g | 2g | 1g | 1g |
| 100 |
| 3g |
| 44 |
| x |
| 100 |
| 3g |
| 44 |
| x |
| 100 |
| 2g |
| 73 |
| y |
| 100 |
| 2g |
| 111 |
| z |
| 100 |
| 2g |
| 44 |
| w |
| 1.46g |
| 10g |
| 2.22g |
| 10g+2g-0.88g+8.88g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | �� | �� | �� | �� | �� |
| ���� | ʳ�� | �� | ����ˮ | ʯ��ˮ | ������Һ |
| ��Ҫ�ɷ� | CH3COOH | C2H5OH | C12H22O11 | Ca��OH��2 | Na2CO3 |
| ��Һ��pH | 3 | 7 | 7 | 11 | 8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ��ͬѧ | ��ͬѧ |
| ���顢������������ | ��һ�飺���顢������������ˮ | ��һ�飺������������ |
| ��������ˮ | �ڶ��飺������ | �ڶ��飺���顢������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
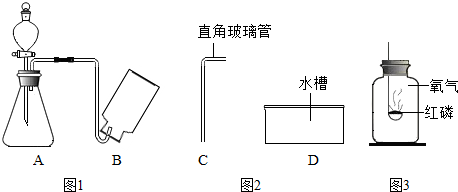
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
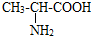 �������ǹ��ɵ����ʵĻ�����λ�����Ļ�ѧʽ��C3H702N�����й��ڱ������������ȷ���ǣ�������
�������ǹ��ɵ����ʵĻ�����λ�����Ļ�ѧʽ��C3H702N�����й��ڱ������������ȷ���ǣ�������| A����������̼���⡢����������ԭ�ӹ��� |
| B�������������̼Ԫ������Ԫ�ص�������Ϊ9��8 |
| C��һ������������к���48������ |
| D������������Ԫ�ص������������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com