
��4��26�գ�ȫʡ����ơ��������ҵ��ơ�����γǹ�˾20��ǧ����ơ��Ŀ��ʽͶ��������
�ٹ�˾��ʽ�ij�����Ʈ����������ѿ��ζ�����ŵ���ζ˵�� ��
A�����Ӻ�С B.���Ӽ��м�϶ C.������Ϊ���˶� D.���ӿ��Էֳ�ԭ��
��ơ����������У���ĸ�ǡ�ħ��ʦ�����ܽ���ѿ֭ת��Ϊ�ƾ��Ͷ�����̼���ñ仯���� �仯��ѡ���������ѧ��������ĸ�������� ![]() ��
��
��ơ��������Ҫ������ȥ�����ε���ˮ��ʵ���ҳ��� �Լ�������ˮ��Ӳˮ��
��ơ�Ƴ����ԣ����ڵ�ơ�ƿ���������ϲ�Ứ�ܡ��ⶨơ�Ƶ����ȿ��� ��
��ơ�����������ʱҪ��ֹ���Ⱪɹ��ԭ���� ��
��ȥ���������������иɺ����ꡣ��ũҵ�����ϣ���0.1%���Ȼ�����Һ��1��1����5��6Сʱ���֣�������������ڸɺ������µij�ѿ�ʡ�
��������20kg0.1%���Ȼ�����Һ���֣���Ҫ�Ȼ��� kg��
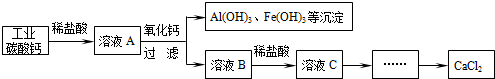
���Թ�ҵ̼��ƣ���������Na+��Al3+��Fe3+�����ʣ������Ȼ��Ƶ���Ҫ�������£�

̼�����ϡ���ᷴӦ�Ļ�ѧ����ʽΪ ������ҺB�м���ϡ����������� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
. |
| ||
. |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com