


科目:初中化学 来源: 题型:


查看答案和解析>>
科目:初中化学 来源: 题型:

查看答案和解析>>
科目:初中化学 来源: 题型:
某兴趣小组的同学探究浓硫酸与铁定(碳素钢)反应后产生气体的成分.
【查阅资料】①Fe与浓H2SO4反应,加热,有SO2生成
②C与浓H2SO4反应,加热,有CO2和SO2生成
③SO2可使澄清石灰水变浑浊
④SO2可使品红溶液的红色褪去,而CO2不能
⑤SO2可与酸性KMnO4溶液反应使其褪色,而CO2不能
⑥碱石灰的主要成分为NaOH和Cao
【实验探究】
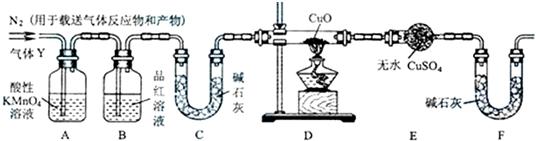
(1)称取24.0g铁钉放入60.0mL浓硫酸中,加热,充分反应后得到的溶液X并收集到气体Y.甲同学通过实验测定并推知气体Y中SO2气体的体积分数为66.7%.同学们认为气体Y中还可能还有H2和Z气体,则Z气体可能是_______.探究实验的主要装置如图所示:

(2)装置A中试剂的作用是____________.
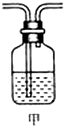
(3)为确认Z的存在,需要将装置甲(如图所示)接入上述装置的(填编号) 之间.装置甲中的液体名称是 .

(4)实验中,如果观察到装置D中 ,且装置E中 ,则可确认Y中还有H2气体.
(5)如果需要测定限定体积气体Y中H2的含量(H2约为0.01g).除可用测量H2体积方法外, (选填:“能”或“不能”)用称量上图中装置D、装置E的质量变化的方法,通过计算说明你判断的理由.
查看答案和解析>>
科目:初中化学 来源: 题型:
查看答案和解析>>
科目:初中化学 来源:2011年江苏省南京市中考化学试卷(解析版) 题型:解答题


查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com