ЈЁ2006?ҪЁЪюЗшТ»ДЈЈ©ТФПВКЗіЈјыөДКөСйЧ°ЦГНј

ЈЁ1Ј©НјЦРУРұкәЕөДТЗЖчГыіЖКЗЈәўЩ
ҫЖҫ«өЖ
ҫЖҫ«өЖ
ўЪ
іӨҫұВ©¶·
іӨҫұВ©¶·
ЈЁ2Ј©ЦЖИЎІўКХјҜёЙФпөДCO
2ЖшМеЈ¬СЎУГөДЧ°ЦГЧйәПКЗ
BDH»тBFH
BDH»тBFH
ЈЁМоЧ°ЦГЧЦДёҙъәЕЈ¬ПВН¬Ј©Ј¬јмСйКЗ·сКХјҜВъөД·Ҫ·ЁКЗ
Ҫ«ИјЧЕөДДҫМх·ЕФЪЖҝҝЪЈ¬№ЫІмКЗ·сПЁГр
Ҫ«ИјЧЕөДДҫМх·ЕФЪЖҝҝЪЈ¬№ЫІмКЗ·сПЁГр
Ј®ЦЖИЎёГЖшМеөД»ҜС§·ҪіМКҪКЗ
CaCO3+2HClЁTCaCl2+H2O+CO2Ўь
CaCO3+2HClЁTCaCl2+H2O+CO2Ўь
Ј»РЎГчҪЁТйҪ«ПЎСОЛб»»іЙЕЁСОЛбЈ¬ДгИПОӘҙЛ·Ҫ·ЁҝЙРРВрЈҝ
І»ҝЙРР
І»ҝЙРР
ЈЁМоЎ°ҝЙРРЎұ»тЎ°І»ҝЙРРЎұЈ©Ј¬АнУЙКЗ
ЕЁСОЛбҫЯУР»У·ўРФЈ¬К№CO2ЦР»мИлHClЖшМе
ЕЁСОЛбҫЯУР»У·ўРФЈ¬К№CO2ЦР»мИлHClЖшМе
Ј®
ЈЁ3Ј©Ді»ҜС§СРҫҝРФРЎЧйөДͬѧѡУГЙПКцЧ°ЦГ¶ФіЈјы»Ҝ·КҪшРРБЛМҪҫҝЈә
МјЛбЗвп§ЈЁNH
4HCO
3Ј©КЗТ»ЦЦіЈјыөД
өӘ
өӘ
·КЈ¬КЬИИТЧ·ЦҪвЙъіЙИэЦЦ»ҜәПОпЈ¬ЖдЦРТ»ЦЦКЗ°ұЖшЈЁNH
3Ј©Ј¬БнБҪЦЦҝЙДЬКЗ
CO2
CO2
Ўў
H2O
H2O
Ј»ОӘБЛЦӨГчҙЛІВПлЈ¬СЎФсЙПКцІҝ·ЦЧ°ЦГЈ¬ёчҪУҝЪөДБ¬ҪУЛіРтКЗaҪУ
h
h
ҪУ
i
i
ҪУ
g
g
Ј®
ЈЁ4Ј©ИзУГH
2O
2әНMnO
2ЦЖСхЖшЈ¬УҰСЎУГ
B
B
ЧчОӘ·ўЙъЧ°ЦГЈ¬Жд»ҜС§·ҙУҰ·ҪіМКҪОӘ
Ј®
¶ФҙЛКөСйЈ¬ИГОТГЗјМРшЛјҝјІўСРҫҝјёёцОКМвЈә
ОКМвўЩЈәҙЯ»ҜјБMnO
2өДУГБҝ¶Ф·ҙУҰЛЩВКУРГ»ОЮУ°ПмЈ¬ОТөДКөСй·Ҫ°ёКЗЈәГҝҙОҫщУГ30mL10%өДH
2O
2ИЬТәЈ¬ІЙУГІ»Н¬БҝMnO
2·ЫД©ЧцҙЯ»ҜјБЈ¬Ів¶ЁёчҙОКХјҜөҪ500mLСхЖшКұЛщУГөДКұјдЈ¬Ҫб№ыИзПВЈәЈЁЖдЛыКөСйМхјюҫщПаН¬Ј©
| КөСйҙОРт |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
| MnO2·ЫД©УГБҝЈЁgЈ© |
0.1 |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.7 |
0.8 |
| ЛщУГКұјдЈЁtЈ© |
17 |
8 |
7 |
5 |
4 |
3 |
2 |
2 |
ЗлДг·ЦОцұнЦРКэҫЭ»ШҙрЈәMnO
2өДУГБҝ¶Ф·ҙУҰЛЩВКУРОЮУ°ПмЈ¬Из№ыУРЈ¬ФхСщУ°ПмЈҝ
ҙрЈә
УРУ°ПмЈ¬ФЪТ»¶Ё·¶О§ДЪЈ¬MnO2өДУГБҝФҪҙуЈ¬·ҙУҰФҪҝм
УРУ°ПмЈ¬ФЪТ»¶Ё·¶О§ДЪЈ¬MnO2өДУГБҝФҪҙуЈ¬·ҙУҰФҪҝм
Ј®
ОКМвўЪЈәH
2O
2ИЬТәөДИЬЦКЦКБҝ·ЦКэ¶Ф·ҙУҰЛЩВКУРГ»УРУ°ПмЈ¬ДгөДКөСй·Ҫ°ёКЗЈә
ҙрЈә
ГҝҙОУГөИБҝөДMnO2Ј¬ІЙУГЧгБҝІ»Н¬ИЬЦКЦКБҝ·ЦКэөДH2O2ИЬТәҪшРРКөСйЈ¬№ЫІмКХјҜөИБҝЖшМеКұЛщПыәДөДКұјд
ГҝҙОУГөИБҝөДMnO2Ј¬ІЙУГЧгБҝІ»Н¬ИЬЦКЦКБҝ·ЦКэөДH2O2ИЬТәҪшРРКөСйЈ¬№ЫІмКХјҜөИБҝЖшМеКұЛщПыәДөДКұјд
Ј®
ОКМвўЫЈә»№УРДДР©ТтЛШҝЙДЬУ°ПмёГ·ҙУҰөДЛЩВКДШЈҝ
ЗлЛөіцДгөДТ»ёцІВПлЈә
MnO2ҝЕБЈҙуРЎУР№Ш
MnO2ҝЕБЈҙуРЎУР№Ш
Ј®
ОКМвўЬЈәТСЦӘСхЖшФЪёГКөСйМхјюПВөДГЬ¶ИОӘ1.28g/LЈ¬ИфТӘЦЖИЎ250mLСхЖшЈ¬јЖЛгАнВЫЙПЦБЙЩРиТӘПыәД30%өДH
2O
2ИЬТә¶аЙЩҝЛЈҝЈЁПа¶ФФӯЧУЦКБҝЈәH-1O-16Ј©



 ФД¶БҝміөПөБРҙр°ё
ФД¶БҝміөПөБРҙр°ё

 ·ЫұККЗТ»ЦЦіЈУГөДҪМС§УГЖ·Ј®ТСЦӘЖдЦчТӘіЙ·ЦЦРә¬УРёЖФӘЛШЈ¬ДіКөСйРЎЧйөДН¬С§¶Ф·ЫұКөДЧйіЙТФј°АыУГ·ЫұКҪшРРУР№ШМҪҫҝЈә
·ЫұККЗТ»ЦЦіЈУГөДҪМС§УГЖ·Ј®ТСЦӘЖдЦчТӘіЙ·ЦЦРә¬УРёЖФӘЛШЈ¬ДіКөСйРЎЧйөДН¬С§¶Ф·ЫұКөДЧйіЙТФј°АыУГ·ЫұКҪшРРУР№ШМҪҫҝЈә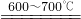 FeO+CO2
FeO+CO2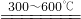 2Fe3O4+CO2
2Fe3O4+CO2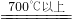 Fe+CO2
Fe+CO2 Ј»ИфТФФӯ№ММеЦРСхФӘЛШөДЧӘ»ҜАҙұнҙп·ҙУҰөДЧӘ»ҜВКФтёГ·ҙУҰЧӘ»ҜВКОӘ________Ј®ЈЁҪб№ыУГ°Щ·ЦКэұнКҫЈ¬ұЈБфТ»О»РЎКэЈ©Ј®
Ј»ИфТФФӯ№ММеЦРСхФӘЛШөДЧӘ»ҜАҙұнҙп·ҙУҰөДЧӘ»ҜВКФтёГ·ҙУҰЧӘ»ҜВКОӘ________Ј®ЈЁҪб№ыУГ°Щ·ЦКэұнКҫЈ¬ұЈБфТ»О»РЎКэЈ©Ј®