
ijУ����С�飬���ⶨһ�����ο������Ҷ��ڿ����ж�����̼��������������趴�ڿ����в�����������̼��������������壩������������ʵ�飺
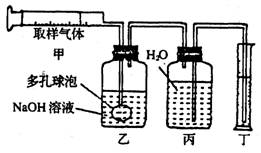
(1)ȡ�������������Ҷ��ĵײ�ȡ������������ù��ƿ�Ӷ���ȡ��һƿ������Ʒ�ķ����� ��
(2)�ⶨ��150mL��ע�����ӹ��ƿ�г�ȡ100mL����������ͼ��ʾ��װ�ý���ʵ�飻
����װ����NaOH��Һ�����ն�����̼���壬��ʵ���������Ͳ��ˮ�����Ϊ99mL���������� ����������������д��
A��������̼ B�������������Ļ������ C�������г�������̼�����������
(3)���㣺�������ж�����̼���������Ϊ ��
(4)ΪӪ����õ����λ��������Ҷ����οͿ���������ΪӦ��ȡ��Щ��ʩ�������Ҷ��ڶ�����̼�ĺ����� ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУ����С�飬���ⶨ���������Ҷ��ڿ�����CO2��������������趴�ڿ����в�����CO2��������������壩����������ͼʵ�飺
ijУ����С�飬���ⶨ���������Ҷ��ڿ�����CO2��������������趴�ڿ����в�����CO2��������������壩����������ͼʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ����С�飬���ⶨһ�����ο������Ҷ��ڿ����ж�����̼��������������趴�ڿ����в�����������̼��������������壩������������ʵ�飺

(1)ȡ�������������Ҷ��ĵײ�ȡ������������ù��ƿ�Ӷ���ȡ��һƿ������Ʒ�ķ����� ��
(2)�ⶨ��150mL��ע�����ӹ��ƿ�г�ȡ100mL����������ͼ��ʾ��װ�ý���ʵ�飻
����װ����NaOH��Һ�����ն�����̼���壬��ʵ���������Ͳ��ˮ�����Ϊ99mL���������� ����������������д��
A��������̼ B�������������Ļ������ C�������г�������̼�����������
(3)���㣺�������ж�����̼���������Ϊ ��
(4)ΪӪ����õ����λ��������Ҷ����οͿ���������ΪӦ��ȡ��Щ��ʩ�������Ҷ��ڶ�����̼�ĺ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡ̩���г��б�ҵ��ѧҵ���Ի�ѧģ���Ծ������� ���ͣ���ѡ��
ijУ����С�飬���ⶨһ�����ο������Ҷ��ڿ����ж�����̼��������������趴�ڿ����в�����������̼��������������壩������������ʵ�飺
(1)ȡ�������������Ҷ��ĵײ�ȡ������������ù��ƿ�Ӷ���ȡ��һƿ������Ʒ�ķ����� ��
(2)�ⶨ��150mL��ע�����ӹ��ƿ�г�ȡ100mL����������ͼ��ʾ��װ�ý���ʵ�飻
����װ����NaOH��Һ�����ն�����̼���壬��ʵ���������Ͳ��ˮ�����Ϊ99mL���������� ����������������д��
A��������̼ B�������������Ļ������ C�������г�������̼�����������
(3)���㣺�������ж�����̼���������Ϊ ��
(4)ΪӪ����õ����λ��������Ҷ����οͿ���������ΪӦ��ȡ��Щ��ʩ�������Ҷ��ڶ�����̼�ĺ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ�꽭��ʡ�������ٴ����о��꼶���ϣ��ڶ����¿���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com