
(11��)������������װ�ã��������������ʵ��������ѡ���������˵�����װ�ã����ش��й����⣺��ÿ��װ�������ظ�ѡ�ã�ÿ��װ���ڵ�ҩƷ���������ģ�
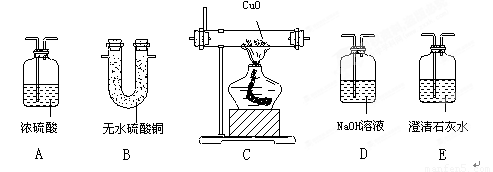
��1��[ʵ��һ]ij������ܺ��� �������е�һ�ֻ��֣���ͨ���۲�ʵ���������о����������ɣ�
�������е�һ�ֻ��֣���ͨ���۲�ʵ���������о����������ɣ�
�� ��ѡ�������װ�ü��������Ⱥ�����˳���ǣ������� B
B
 E����װ�ô�����д��ȷ��˳���ظ�ʹ�ã���
E����װ�ô�����д��ȷ��˳���ظ�ʹ�ã���
�� �������������Ⱥ�����˳�����õ�һ��Bװ�õ�Ŀ����_______________������Eװ�õ�Ŀ����_______________; .
��2��[ʵ���]ij�������л����������г��ȼ�պ���ֻ��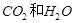 �������������壬Ӧ��ȼ�պ�õ�����������ͨ��_____________����дװ�õĴ��ţ������ⶨ���л������ɡ������л�����ȼ��ʱ��ȥ��4.8���������Ⱥ����ӵ�����װ����ʵ����ʵ��ǰ�����ֱ�������3.6�˺�4.4�ˣ�����л������ɣ�дԪ�ط��ţ�___________��ɵģ���ӦԪ�ص�������Ϊ_____________��д����������ȣ�����д���������
�������������壬Ӧ��ȼ�պ�õ�����������ͨ��_____________����дװ�õĴ��ţ������ⶨ���л������ɡ������л�����ȼ��ʱ��ȥ��4.8���������Ⱥ����ӵ�����װ����ʵ����ʵ��ǰ�����ֱ�������3.6�˺�4.4�ˣ�����л������ɣ�дԪ�ط��ţ�___________��ɵģ���ӦԪ�ص�������Ϊ_____________��д����������ȣ�����д���������
1��EDACB���ߵ����÷֣��˿�2�֣���
2���������������Ƿ���ˮ�����������������Ƿ���CO2����Cװ�ý�ϣ��жϻ�������Ƿ���CO
3��BD���ߵ����÷֣���C��H��O��3��1��4
������̣�C��4.4*12/44=1.2g ��1�֣� H��3.6*2/18=0.4g(1��)
O����4.4-1.2��+��3.6-0.4��-4.8=1.6g(1��)
C��H��O=1.2��0.4��1.6=3��1��4
��������������ˮ����ͭ��ˮ������������̼��ʹ�����ʯ��ˮ����ǣ�����������ͭ����ˮ��ͭ��һ����̼������ͭ����ͭ�Ͷ�����̼��Ũ��������ˮ�ԣ����з������
Ũ������и���������ã�������ˮ�֣���������3.6g����Ϊˮ���������������Ԫ�ص�������ʯ��ˮ���������������壬��������4.4g����Ϊ������̼�������������̼Ԫ�ص���������Ԫ�ص���������ˮ����Ԫ�ص��������϶�����̼����Ԫ�ص�������ȥȼ��ʱ���˵��������������������ӦԪ�ص�������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)��������������װ�ã���ش��������⣺

|
��2������(H2C2O4) ������Ũ���������·�����Ӧ: H2C2O4======CO2��+CO��+H2O�����ø÷�Ӧ����ȡCO������װ��Ӧѡ ������ĸ������ȥ���е�CO2��ѡ����AC��C��װ����Լ������ ����Сд��ĸ���� a. �ռ���Һ b. Ũ���� c. ����ʯ��ˮ��
��3����ȻCOǰӦ���еIJ����� ������CO��ԭ����ͭ��ĩ��Ӧѡװ�� ������ĸ�����䷴Ӧ����ʽΪ ��
��4����ͼΪij�֡��͡�ʵ��װ�á����G��װϡ���ᣬH����������м������ȴ�������Ϊ ���������ϵιܡ��൱����ͼʵ��װ���е� ������ĸ����������������ʵ�飬���Լ������������⣬�����ܾ��е��ŵ��� ��д1�㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭���Ͼ�ѧ�����ר��ѧУ���꼶5�½β��Ի�ѧ������������ ���ͣ������
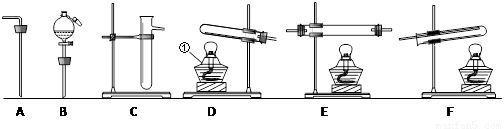
(11��)������������װ�ã��������������ʵ��������ѡ���������˵�����װ�ã����ش��й����⣺��ÿ��װ�������ظ�ѡ�ã�ÿ��װ���ڵ�ҩƷ���������ģ�
��1��[ʵ��һ]ij������ܺ���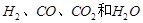 �������е�һ�ֻ��֣���ͨ���۲�ʵ���������о����������ɣ�
�������е�һ�ֻ��֣���ͨ���۲�ʵ���������о����������ɣ�
����ѡ�������װ�ü��������Ⱥ�����˳���ǣ������� B
B
 E����װ�ô�����д��ȷ��˳���ظ�ʹ�ã���
E����װ�ô�����д��ȷ��˳���ظ�ʹ�ã���
�ڰ������������Ⱥ�����˳�����õ�һ��Bװ�õ�Ŀ����_______________������Eװ�õ�Ŀ����_______________; .
��2��[ʵ���]ij�������л����������г��ȼ�պ���ֻ��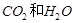 �������������壬Ӧ��ȼ�պ�õ�����������ͨ��_____________����дװ�õĴ��ţ������ⶨ���л������ɡ������л�����ȼ��ʱ��ȥ��4.8���������Ⱥ����ӵ�����װ����ʵ����ʵ��ǰ�����ֱ�������3.6�˺�4.4�ˣ�����л������ɣ�дԪ�ط��ţ�___________��ɵģ���ӦԪ�ص�������Ϊ_____________��д����������ȣ�����д���������
�������������壬Ӧ��ȼ�պ�õ�����������ͨ��_____________����дװ�õĴ��ţ������ⶨ���л������ɡ������л�����ȼ��ʱ��ȥ��4.8���������Ⱥ����ӵ�����װ����ʵ����ʵ��ǰ�����ֱ�������3.6�˺�4.4�ˣ�����л������ɣ�дԪ�ط��ţ�___________��ɵģ���ӦԪ�ص�������Ϊ_____________��д����������ȣ�����д���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��ȫ���п�����ר����ר���� ̽���⣨һ�� ���ͣ�̽����
(12��)��������������װ�ã���ش��������⣺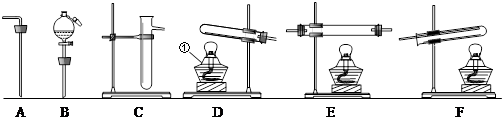
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��ȫ���п�����ר����ר����̽���⣨һ�� ���ͣ�̽����
(12��)��������������װ�ã���ش��������⣺
|
��2������(H2C2O4) ������Ũ���������·�����Ӧ: H2C2O4======CO2��+CO��+H2O�����ø÷�Ӧ����ȡCO������װ��Ӧѡ ������ĸ������ȥ���е�CO2��ѡ����AC��C��װ����Լ������ ����Сд��ĸ���� a. �ռ���Һ b. Ũ���� c. ����ʯ��ˮ��
��3����ȻCOǰӦ���еIJ����� ������CO��ԭ����ͭ��ĩ��Ӧѡװ�� ������ĸ�����䷴Ӧ����ʽΪ ��
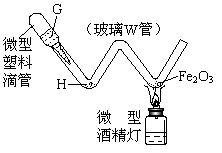
��4����ͼΪij�֡��͡�ʵ��װ�á����G��װϡ���ᣬH����������м������ȴ�������Ϊ ���������ϵιܡ��൱����ͼʵ��װ���е� ������ĸ����������������ʵ�飬���Լ������������⣬�����ܾ��е��ŵ��� ��д1�㣩��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com